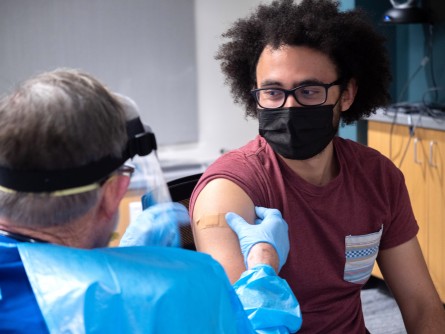
Richard Biggs, 20, an evolutionary biology major at the University of Colorado Boulder gets his first dose of the Moderna vaccine from Dr. Laird Wolfe. Biggs is one of the first group of students in a new study to determine if the vaccine can prevent COVID-19 infection and transmission in the student population. CU Boulder students will join 12,000 other college students in the country. Courtesy of Glenn Asakawa/University of Colorado
The COVID-19 Prevention Network (CoVPN), headquartered at Fred Hutchinson Cancer Research Center, today announced the launch of Prevent COVID U, a new study evaluating SARS-CoV-2 infection and transmission among college students vaccinated with the Moderna COVID-19 vaccine, mRNA-1273.
The trial is funded by the Federal COVID-19 Response Program and the National Institute of Allergy and Infectious Diseases (NIAID). It is designed to determine if the mRNA-1273 vaccine, currently authorized for emergency use by the U.S. Food and Drug Administration, can prevent infection with SARS-CoV-2 (including asymptomatic infection), limit virus in the nose, and reduce transmission of the virus from vaccinated persons to their close contacts.
“This study builds on the Phase 3 COVID-19 clinical trials that tested the ability of vaccines to prevent symptomatic and severe COVID-19 disease in adults. The new trial will tell us whether a person can become infected after they’ve been vaccinated and if the vaccine will stop the virus from spreading person-to-person,” said Dr. Larry Corey, principal investigator of CoVPN’s operations program, professor at Fred Hutchinson Cancer Research Center, and one of the study leaders. “The answers to these questions have implications for public health and will allow us to make more science-based decisions about mask use and social distancing post-vaccination – especially when new variants are emerging.”
The Prevent COVID U study is a randomized, open-label, controlled study. Investigators will enroll approximately 12,000 college students aged 18-26 years at more than 20 universities across the U.S. and follow them over a five-month period. The study is funded by NIAID, part of the National Institutes of Health (NIH).
Large numbers of SARS-CoV-2 infections have been reported on campuses throughout the U.S. A nationwide survey found that more than 397,000 infections were counted at 1,800-plus universities after reopening in the fall of 2020. Two separate studies in the CDC’s Morbidity and Mortality Weekly Report last October reported that SARS-CoV-2 infections among young people aged 18-22 increased 55% nationally between August and September 2020. Between June and August 2020, young people aged 20-29 had the highest incidence of SARS-CoV-2 infection in the U.S. – accounting for more than 20% of all confirmed cases.
“High-density housing, the impulse to socialize and less fear of severe disease in young people are all factors that contribute to the high burden of SARS-CoV-2 infection on college campuses,” said Dr. Holly Janes, a professor at Fred Hutch and one of the leaders of the study.
Dr. Elizabeth Brown, a professor at Fred Hutch and a study leader who worked closely with Janes in designing the trial, agreed, noting that, “College campuses are model settings for studying transmission of infection, given that they are relatively closed populations.”
Prevent COVID U opened initial study sites March 25. It is a two-arm trial – half of the students will be randomly selected to receive the vaccine right away at enrollment, while the other half will get the vaccine four months later. All participants will know which arm of the trial they are in at enrollment and all will ultimately receive the vaccine. Throughout the study period, participants will complete questionnaires via an eDiary app, swab their nose daily for COVID-19 infection, and provide periodic blood samples.
Because testing the vaccine’s effectiveness to reduce and/or prevent transmission requires measuring spread of the virus to others, about 25,500 individuals identified by participants in the main study as “close contacts” also will be invited to take part in the trial. Close contacts who have agreed to participate in the study will be asked to answer weekly questionnaires via eDiary, provide two blood samples and take daily swabs of their nose for two weeks.
“Our hope is that we demonstrate that COVID-19 vaccines prevent people from getting infected with coronavirus in the first place and that it stops transmission to others,” said Dr. Corey. “The emphasis on following close contacts among those students who acquire COVID-19 in the trial is one of the unique aspects of the trial.”
The universities participating are:
Charles Drew University
Clemson University
Indiana University – Bloomington
Morehouse School of Medicine
Northwestern University
Stony Brook University
Texas A&M – College Station
Texas A&M – Kingsville
University of Arizona
University of California – San Diego
University of Colorado – Boulder
University of Florida – Gainesville
University of Illinois – Urbana-Champaign
University of Kentucky
University of Maryland – College Park
University of Nebraska
University of North Carolina
University of Virginia
University of Washington
Wake Forest
West Virginia University
Winston-Salem State University
Source: Covid-19 Prevention Network (CoVPN)
Be the first to comment on "Clinical Trial Will Test If COVID-19 Vaccine Prevents Infection and Spread of SARS-CoV-2 Among College Students"