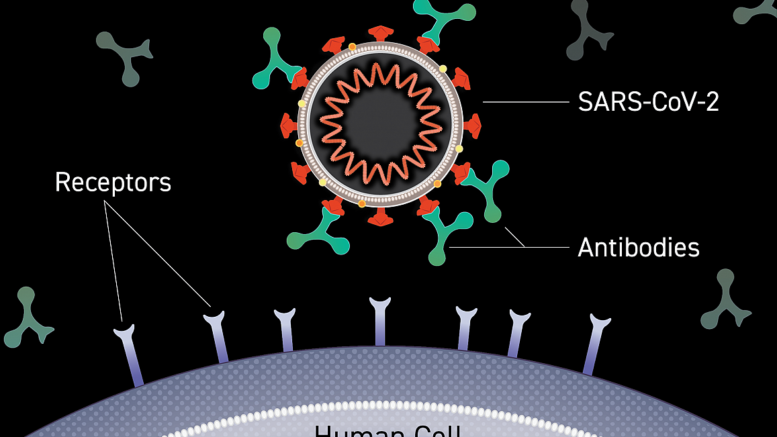
Image of an antibody binding to the surface of a virus, blocking entry into a human cell. Courtesy of Lisa Donohue, CoVPN
Two Phase 3, randomized, placebo-controlled, double-blind clinical trials testing whether experimental monoclonal antibodies (mAbs) can prevent infection by SARS-CoV-2 coronavirus are now enrolling healthy adults at clinical trial sites in the United States. Many of the trial sites and study investigators are part of the COVID-19 Prevention Network(link is external) (CoVPN), recently established by the National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes of Health. SARS-CoV-2 is the virus that causes coronavirus disease 2019 (COVID-19). The trials are enrolling adults who are at risk of infection due to close contact at work or home to persons with SARS-CoV-2 infection.
“The COVID-19 Prevention Network is designed to conduct large-scale trials rapidly and efficiently,” said NIAID director Anthony S. Fauci, MD. “This network will allow us to test the safety and efficacy of monoclonal antibodies and other preventive measures to help identify how best to reduce the level of SARS-CoV-2 infection and ultimately end the COVID-19 pandemic.”
Monoclonal antibodies(link is external) are laboratory-made versions of proteins naturally produced by the immune system in response to invading viruses or other pathogens. Neutralizing antibodies, whether natural or monoclonal, can bind directly to portions of viruses that they use to attach to and enter cells, preventing them from initiating the infection cycle. Monoclonal antibodies may provide short-term protection from SARS-CoV-2 and could serve as important components of the COVID-19 pandemic response until vaccines become available.
One trial is being conducted jointly by NIAID and trial sponsor Regeneron Pharmaceuticals of Tarrytown, NY. It will evaluate Regeneron’s investigational double mAb combination, REGN-COV-2, which is designed to bind to two points on the SARS-CoV-2 spike protein and prevent it from entering healthy cells. The trial will enroll approximately 2,000 asymptomatic adults who are household contacts of persons with SARS-CoV-2 infection. Participants must have been in close contact (typically due to residing at the same address) with the infected person in a 96-hour window preceding administration of either REGN-CoV-2 or placebo. In addition to assessing safety, the trial will seek to define whether REGN-COV-2 can prevent infection or disease symptoms in those already infected. The efficacy assessment will be a one-month period following administration of REGN-COV-2 or placebo. All trial participants will be followed for safety for seven months after efficacy assessment period ends.
Additional details about this trial are available at clinicaltrials.gov using the identifier NCT04452318. Interested participants can also visit the CoVPN website(link is external) for details. Doctors or potential participants may also contact the sponsor’s Clinical Trials Administrator at 844-734-6643 or clinicaltrials@regneneron.com(link sends e-mail) for information on enrolling.
A second trial, sponsored by Eli Lilly and Company of Indianapolis, Indiana, and implemented in collaboration with NIAID, will evaluate LY-CoV555, a mAb isolated from a recovered COVID-19 patient by scientists at AbCellera (Vancouver, British Columbia, Canada) and the NIAID Vaccine Research Center, and developed by Eli Lilly and Company. This trial will assess whether LY-CoV555 can prevent SARS-CoV-2 infection among people at high risk of exposure due to residing or working in skilled nursing or assisted living facilities. Within one week of identification of a case of SARS-CoV-2 infection at a facility, study investigators will enroll trial volunteers and evaluate the prevention efficacy and safety of LY-CoV555, compared to placebo, over an 8-week period. The trial will also evaluate efficacy in preventing symptoms of a given severity in those already infected. Participants will continue to be followed for safety for an additional 16 weeks. Up to 2,400 participants will be randomized to receive intravenous infusion of either LY-CoV555 or placebo.
Additional information about this trial is available at clinicaltrials.gov using the identifier NCT04497987. Clinical investigators, hospitals or clinical sites interested in participating in one of Lilly’s clinical trials for a potential COVID-19 treatment, should call 1-877-CT-LILLY (1-877-285-4559) or email covid19potentialsite@lilly.com
Source: National Institutes of Health (NIH)
When I originally commented I seem to have clicked the -Notify me when new comments are added- checkbox and now each time a comment is added I recieve four emails with the same comment. Perhaps there is a means you can remove me from that service? Many thanks!
I loved as much as you will receive carried out right here. The sketch is tasteful, your authored subject matter stylish. nonetheless, you command get bought an edginess over that you wish be delivering the following. unwell unquestionably come further formerly again since exactly the same nearly a lot often inside case you shield this increase.
Hey very interesting blog!
Pretty great post. I simply stumbled upon your blog and wanted to mention that I have really enjoyed browsing your blog posts. In any case I’ll be subscribing for your feed and I am hoping you write again soon!
Appreciating the time and energy you put into your site and in depth information you present. It’s awesome to come across a blog every once in a while that isn’t the same out of date rehashed material. Fantastic read! I’ve saved your site and I’m including your RSS feeds to my Google account.
Howdy! I know this is somewhat off-topic but I had to ask. Does running a well-established blog like yours take a massive amount work? I’m completely new to operating a blog but I do write in my diary everyday. I’d like to start a blog so I will be able to share my experience and thoughts online. Please let me know if you have any ideas or tips for new aspiring bloggers. Appreciate it!
Pretty great post. I simply stumbled upon your blog and wanted to mention that I have really enjoyed browsing your blog posts. In any case I’ll be subscribing on your feed and I hope you write again soon!
Hey just wanted to give you a quick heads up and let you know a few of the images aren’t loading correctly. I’m not sure why but I think its a linking issue. I’ve tried it in two different browsers and both show the same results.
I think this is one of the most important information for me. And i’m glad reading your article. But wanna remark on few general things, The website style is great, the articles is really nice : D. Good job, cheers
I’m really enjoying the design and layout of your site. It’s a very easy on the eyes which makes it much more enjoyable for me to come here and visit more often. Did you hire out a designer to create your theme? Outstanding work!