Are Masks Really Not Working or Do We Need to Focus on Community-level Masking Education Around Proper Handling and Indications for Use?
By Shanina Knighton, PhD, RN, CIC
This article originally appeared in the March 2024 issue of Healthcare Hygiene magazine.
As we reflect on being what some people would describe as being out of the “eye of the storm” as it relates to COVID-19, there are many opinions and reflections about what worked well and what did not. One controversial scientific question that still exists is “are masks effective?” A few scientific reviews, including Cochrane1 suggest the effectiveness of masks were inconclusive, whereas mainstream media outlets shared the message that “masks do not work.”
As an infection preventionist, nurse and scientist, I understand that non-healthcare workers do not always understand how important proper wear and handling of masks are and the risks associated with mishandling and reuse2 of disposable masks. In healthcare, it is established that if masks are mishandled or improperly used, they can increase the risk for infection transmission between patients. For example, in the pre-pandemic era it was standard for surgical masks to be one-time use when needed for precautions and or if you had a patient that required airborne precautions you would be told to use a N-95 mask for just that patient during your shift. These measures helped decrease the risks of transmission. During the pandemic, emergency reuse was emphasized due to limited supplies favoring the opinion that mask reuse provided more of an upside for protection than risks. However, data was scarce in determining that.
As attention turns more to public health and community-level infection prevention and control efforts it intreats the scientific community to pause and ask, “Are we educating and measuring the effectiveness of basic infection prevention and control practices and in this example— mask use and handling including the practical uses that can keep everyday people safe. Furthermore, do we educate the community on the benefits of proper mask use and handling and the potential consequences associated with misuse and mishandling?
Briefly, I summarize some examples of misuse and education tips often overlooked.
Dirty hands taking the mask on and off parallels to a dirty mask. Hand hygiene is the simple most important wat to prevent the spread of germs that lead to infections. During the pandemic people were told to put on masks. However, evaluating if people clean their hands at all, less known correctly before and after putting their masks on should be considered. Mouths and noses expel germs and are also entry for germs. The mask coming in direct contact with the mouth and nose means that people run the risk of unknowingly transmitting germs directly to and from their face by way of the mask.
Dirty cell phones hashtag our third hand links to a dirty mask. Cell phones are seen as the third hand and are shown to be 10 times dirtier 3than a toilet seat. Cell phones create a segway to germs being directly transmitted to the face. Imagine your face, cell phone and mask all coming in contact with each other as you place your phone against your mask while keeping it on or pulling it down to take a call instead of removing it from ear to ear. Cell phones can get contaminated just because we use them for almost everything. People that do bathroom scrolling are at risk given many studies showing the splash effect from sinks4 and toilets5 thus justifying the transmission of some harmful germs. Mobile hygiene and touchscreen hygiene is important to avoiding germs that can travel to or from your “third hand.”
Mishandling and mis-wearing of masks connect to microbes. Treading through the hallways in many walks of life from emergency departments where the highest foot traffic occurs to COVID testing or vaccine clinics many people including healthcare workers could be observed time and time again wearing masks as chin girdles or beneath their nose while some wore them correctly above the bridge of their nose with a snug fit. Incorrect masking wearing means that the protection against particulates is inaccurate compared to what the manufacturer’s protection standards might say. Many factors such as not removing masks from ear to ear, sitting masks on unclean surfaces, wearing disposable masks multiple times because it is not “visibly” dirty and exposure of the mask under certain conditions can alter the effectiveness of how well a mask will protect someone. Education round correct technique and understanding of how to handle and wear masks is needed for community use of masks.
We won’t be masked forever, but we should when it matters. It is not likely people will wear masks while sitting privately in their cars forever. It is also not likely that masking will go away. Geographical areas where transmission is high, working with high-risk populations or being considered a part of a high-risk population warrants consideration for wearing masks according to the Centers for Disease Control and Prevention6. However, based on my expertise there are situations where transmission is at its highest that influences my personal masking habits. While I do not wear masks in every setting, I do wear them on public transportation7 where cleaning practices have slowed, and high air exchange is no longer occurring. I wear masks in old buildings8 where I know the heating, ventilation and air conditioning systems are not up to par. If I am in an elevator9 with strangers, I wear my mask. Unfortunately, you cannot predict when someone will have to cough or sneeze in these types of closed environments therefore, I would rather take my chances on wearing a mask for that short duration of time than coming in contact with droplets that can make me sick. Many agree that wearing masks in healthcare settings should go away, but I disagree. We know that within healthcare settings people are likely to go to facilities because they are sick including a higher likelihood to carry transmissible germs such as respiratory illnesses.
Disposable masks are disposable and do expire.10 It is understandable that resources at certain points during the pandemic were scarce. However, people should be aware that similarly to following directions on a box of food for preparation directions, masks have directions that include country of origin, expiration date and most importantly directions for use including the number of hours that you should wear them. Furthermore, similarly to how technology and food gets recalled, education around ensuring masks are breathable without restriction, how to inspect masks for defects, and proper reporting and discarding if you detect defects is important, yet something rarely considered during evaluation of mask effectiveness.
Notably, many healthcare protocols that are implemented in community settings are derived from healthcare settings as it relates to messaging about hand hygiene and proper mask wearing. While many heard “just wear the mask”— this led to many healthcare professionals observing people mishandle masks. Similar to many Infection Preventionists, we know that emphasis on mask education could have made a difference in COVID-19 outcomes and still can. People’s mistrust and belief that masks do not work can be negated with sharing the risks associated with improper handling, use and by providing education about mask safety.
Shanina Knighton, PhD, RN, CIC, is an associate professor at Case Western Reserve University.
References:
1. Jefferson T, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. (2023) doi:10.1002/14651858.CD006207.pub6.
2. Easwaran V, et al. Examining factors influencing public knowledge and practice of proper face mask usage during the COVID-19 pandemic: a cross-sectional study. PeerJ 12, e16889 (2024).
3. Kõljalg S, et al. High level bacterial contamination of secondary school students’ mobile phones. Germs 7, 73–77 (2017).
4. Fucini G-B, et al. Sinks in patient rooms in ICUs are associated with higher rates of hospital-acquired infection: a retrospective analysis of 552 ICUs. J. Hosp. Infect. 139, 99–105 (2023).
5. Abney SE, et al. Toilet hygiene—review and research needs. J. Appl. Microbiol. 131, 2705–2714 (2021).
6. CDC. Use and Care of Masks. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html.
7. FTA. Using Your Safety Management System (SMS) to Mitigate Infectious Disease and Respiratory Hazard Exposure. https://www.transit.dot.gov/regulations-and-programs/safety/using-your-safety-management-system-sms-mitigate-infectious-disease.
8. Burridge HC, et al. The ventilation of buildings and other mitigating measures for COVID-19: a focus on wintertime. Proc. Math. Phys. Eng. Sci. 477, 20200855.
9. Liu S and Deng Z. Transmission and infection risk of COVID-19 when people coughing in an elevator. Build. Environ. 238, 110343 (2023).
10. Health C for D and R. Face Masks, Barrier Face Coverings, Surgical Masks, and Respirators for COVID-19. FDA (2024).
Isolation: Pondering the Issues
By Carol Calabrese, RN, BS, T-CSCT, CHESP, CIC
This article originally appeared in the February 2024 issue of Healthcare Hygiene magazine.
During clinical rotation I vividly recall one of my fellow classmates passing medications and walking directly into a room in which the patient was on isolation precautions.
Practicing good isolation techniques is important. We saw this especially in 2014 with the risk of Ebola in the United States. Everyone was franticly trying to re-educate their staff on donning and doffing technique of personal protective equipment (PPE). After the emergency was over, staff went back to their old bad habits. I have asked myself, are they old habits or did they never receive proper education? Are we doing enough isolation education for all healthcare workers (HCWs)?
How, where and when is education provided to HCWs in your institution? Infection preventionists (IPs) struggle to provide much-needed content during hospital orientation which has gone from an hour to just 15-30 minutes. If it is not provided in general orientation, then where is it provided? It might be in the unit/department’s orientation; and if it is, who has trained the preceptors in these locations? Are competencies performed?
With the emphasis we place on isolation, we may not be evaluating many of the issues related to caring for patients/residents that need to be in isolation.
I’ve seen a wide range of infractions. Staff wearing the gown backwards, leaving a gap in the front; staff walking out of isolation with a specimen, taking it to the nurses station to send through the pneumatic tube system; water pitchers being filled incorrectly, dietary walking out of a contact isolation room with the menu and then faxing it to dietary at the nurses station, a respiratory therapist in full PPE at the doorway of the room typing on the computer at the mobile work station; the whole endoscopy cart in contact precautions and a CNA doing one-to-one care in contact precautions without PPE, to name a few.
The Centers for Disease Control and Prevention (CDC) provides clear guidance for donning and doffing PPE, how to manage linen and to use certain disposable equipment but does not discuss other activities involved in patient care.
When a staff member is doing one-to-one care, the typical expectation is that they are wearing PPE for an eight- to12-hour shift, minus breaks. I wonder how realistic an expectation this is. A thought I have had but never implemented is to provide the HCW with hospital-provided scrubs and a lab coat. The HCW would wear the scrubs while doing one-to-one, don the lab coat when they leave the room for breaks and place it on a hook outside of the patient’s room upon re-entry. Naturally hand hygiene is very important prior to donning and doffing the lab coat.
Staff are encouraged to identify all supplies that need to be taken into the room to provide care, however, issues arise with items coming out.
When I trained, we were taught to have a “buddy” that would assist us. I went into the isolation room to collect a urine specimen. I collected the specimen and labelled it. My buddy is outside of the room with the specimen bag, holding it open for me to place the specimen into it. They then took the specimen to the nursing station to send it to the laboratory. The outside of the specimen bag is not contaminated using this process. This process can and should be applied to other items coming out of an isolation room.
Evaluating how other departments providing care to the patient in Isolation are managing items used to care for that patient is critical. A nurse or CNA can be their buddy.
Does this take some coordination? Absolutely; however, it also aids in reducing the risk of transmission.
Over the past few years, research has demonstrated that pathogens such as C. difficile and other multidrug-resistant organisms (MRDOs) are being found in non-isolation rooms.
Teska and Gauthier (2021) suggest that IPs may need to consider having environmental services (EVS) personnel disinfect the floors of patients on Contact Precautions. The references cited discuss the transmission of pathogens between rooms via the shoes and hands of the HCWs.
With this evidence, IPs may also want to consider adding shoe covers to the necessary PPE worn for contact precautions.
The CDC’s CDI TAP Assessment Tool (2022) is helpful to evaluate what is being done to minimize the risk of transmission of CDI, however, I believe this should be utilized for all MDROs.
Per the CDC’s report, Antibiotic Resistance Threats in the United States, 2019, antimicrobial resistance (AR) report, it states that resistance is growing. Utilizing contact precautions will continue to grow as AR grows in addition to the threat of new and emerging pathogens.
As the draft 2024 transmission-based precautions guidance is released by CDC’s Healthcare Infection Control Practices Advisory Committee (HICPAC), it may be time for us to take a deeper look at isolation technique, implementing the use of additional PPE and components to environmental hygiene.
Carol Calabrese, RN, BS, T-CSCT, CHESP, CIC, is an infection prevention consultant with 30-plus years of experience. Her experience includes many settings, acute to industry with leadership background. Connect with Carol on LinkedIn: linkedin.com/in/carol-calabrese-rn-bs-t-csct-chesp-cic-a09bb216 and visit her website at: www.infectionpreventionconsultant.com
Should We Start Thinking More About Patient Hand Hygiene Education in Healthcare?
By Shanina C. Knighton, PhD, RN, CIC
This article originally appeared in the January 2024 issue of Healthcare Hygiene magazine.
For decades now, healthcare-associated infections continue to be an unyielding ongoing patient safety concern affecting millions of people and causing hundreds of thousands of deaths globally each year. The approach to preventing healthcare-acquired infections (HAIs) differ from most patient safety issues as it has many factors associated with infection prevention and control especially for multidrug-resistant organisms (MDROs), antibiotic-resistant organisms and viruses known as “superbugs” that are difficult to prevent and control.
Most innovative strategies to address HAIs focus specifically on antibiotic use, policy, hospital staff practices (e.g., not routinely cleaning hands) and hospital environmental factors (e.g., lack of extensive cleaning between patients). However, an important contributing factor to HAIs has received far less attention and study — the role of patients’ hands as a source of pathogen transmission in healthcare settings. Uncontended, most would argue that hand hygiene is the single most important practice for anyone to prevent the spread of pathogens that lead to HAIs, however there is less agreement on normalizing patient hand hygiene in healthcare settings. Scientific discovery regarding patients’ hand cleanliness and pathogen transmission is relatively new and emerging unlike decades of mounting evidence to support the healthcare worker transmission and hand hygiene.
Motivated from earlier science, healthcare staff hand hygiene has advanced immensely since the decades between the 1960s and the 1990s, confirming the carriage of pathogens on healthcare staff’s hands. Notably, in some of the same studies that recognized the carriage of pathogens on the hands of healthcare staff, results also showed that patients’ hands and bodies also tested positive for pathogens; furthermore, in some cases, patients were found to be the original carrier of these harmful organisms.1-5
Patients can transfer pathogens to their environment and to healthcare staff, and cross-contaminate themselves as germs are not unidirectional. Therefore, they can be transferred in various ways. Documented evidence shows that patients carry one or more MRDOs on their hands,6-9 proximal areas (trunk), and clothing10 thus increasing the risk of contaminating their environment or cross-contaminating themselves. Once admitted to hospitals, patients are at risk of getting or spreading HAIs simply by inadvertently and unknowingly transmitting pathogens from their hands by making contact with their own devices (some indwelling), dressings, surgical wounds, healing and non-healing ulcerations, IV sites, and orifices, including their mouths through making contact with their food.
Furthermore, patients frequently interact with nurses, doctors, visitors, and other patients, which can also increase risks for getting an HAI. While randomized controlled trials that address decolonization and isolation efforts repeatedly and effectively decreased pathogen transmission outside of the patients’ room and after patients are already infected,11 evidence supports bidirectional relationships between patients’ hand contamination and the contamination for high-touch environmental surfaces.12-15
Surfaces and tools commonly used by staff and patients such as bedside tables,12,16-17 bedrails,18 medical devices,12 or call lights contain MDROs.10,16 Contamination of patients’ personal belongings including cell phones19 and clothing10 also increases their risk of contact with MDROs (20,21). Hence, strategies to prevent growth and colonization of MDROs are needed. Recommendations and conclusions of earlier studies have contended that healthcare staff should clean their hands, but for patients, it was only suggested that patients could be a source of transmission resulting in minimal recommendations for patients to have clean hands.1,5 Accrediting bodies and governing entities acknowledge that patients should have an active role in their care to prevent HAIs; however, patients’ role in infection prevention is still passive. For example, patients are encouraged to ask healthcare staff to clean their hands before providing care, but rarely are educated on cleaning their own hands.
Patient hand hygiene as a quality improvement strategy can help prevent the transmission of pathogens that lead to HAIs while simultaneously promoting patient self-management and patient engagement. Furthermore, providing patients with the opportunity to clean their hands is an underutilized approach that can reduce patients’ hand contamination that can lead to infection. For uncontaminated patients, patient hand hygiene can provide an additional layer of protection for them to prevent predisposition to colonization that leads to HAIs. As viral bacterial and fungal infections increase and we continue to face multi-viral seasons comprised of influenza, SARS-CoV2, respiratory syncytial virus, pneumonia, and others it is timely to update the growing body of literature on the status of patient hand hygiene in hospitals, which can influence how we integrate or implement patient hand hygiene programs in acute care settings and long-term care settings.
Shanina C. Knighton, PhD, RN, CIC, is a nurse-scientist, infection preventionist and an associate professor in the Schools of Nursing and adjunct in biomedical engineering at Case Western Reserve University. Knighton was the Inaugural executive director of APIC’s Center for Research Practice and Innovation where she championed their transformation to advance science and practice. She is known for being able to take infection prevention science and turn it into practical and equitable tools for improvement in various settings. During COVID-19, she provided practical prevention tools and guidelines to community members, small businesses, community organizations and public officials including the state of Ohio, the American Nurses Association, the New York State Board of Education, and others. Her practical tips have appeared in media outlets including local news, Forbes, Fox News, Self magazine, and Modern Woman magazine. In 2022 she was recognized as a Case Western Reserve University Faculty Innovator.
References:
1. Casewell M, Phillips I. Hands as route of transmission for Klebsiella species. Br Med J. 1977 Nov 19;2(6098):1315–7.
2. Casewell MW, Desai N. Survival of multiply resistant Klebsiella aerogenes and other Gram-negative bacilli on fingertips. J Hosp Infect. 1983 Dec 1;4(4):350–60.
3. Gwaltney JM, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978 Apr;88(4):463–7.
4. Gwaltney JM. Rhinoviruses. Yale J Biol Med. 1975 Mar;48(1):17–45.
5. Sanderson PJ, Weissler S. Recovery of coliforms from the hands of nurses and patients: activities leading to contamination. J Hosp Infect. 1992 Jun;21(2):85–93.
6. Sunkesula VCK, Kundrapu S, Knighton S, Cadnum JL, Donskey CJ. A Randomized Trial to Determine the Impact of an Educational Patient Hand-Hygiene Intervention on Contamination of Hospitalized Patient’s Hands with Healthcare-Associated Pathogens. Infect Control Hosp Epidemiol. 2017 Jan 5;1–3.
7. Cao J, Min L, Lansing B, Foxman B, Mody L. Multidrug-resistant organisms on patients’ hands: A missed opportunity. JAMA Intern Med. 2016 May 1;176(5):705–6.
8. Mody L, Krein SL, Saint SK, Min LC, Montoya A, Lansing B, et al. A Targeted Infection Prevention Intervention in Nursing Home Residents with Indwelling Devices. JAMA Intern Med. 2015 May 1;175(5):714–23.
9. Mody L, Washer LL, Kaye KS, Gibson K, Saint S, Reyes K, et al. Multidrug-resistant Organisms in Hospitals: What Is on Patient Hands and in Their Rooms? Clin Infect Dis Off Publ Infect Dis Soc Am. 2019 Apr 13.
10. Kanwar A, Cadnum JL, Thakur M, Jencson AL, Donskey CJ. Contaminated clothing of methicillin-resistant Staphylococcus aureus (MRSA) carriers is a potential source of transmission. Am J Infect Control [Internet]. 2018 Jun 22 [cited 2018 Oct 14];0(0). Available from: https://www.ajicjournal.org/article/S0196-6553(18)30679-5/abstract
11. Peng HM, Wang LC, Zhai JL, Weng XS, Feng B, Wang W. Effectiveness of preoperative decolonization with nasal povidone iodine in Chinese patients undergoing elective orthopedic surgery: a prospective cross-sectional study. Braz J Med Biol Res [Internet]. 2017 Dec 18 [cited 2018 Oct 14];51(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5734184/
12. Pilmis B, Billard-Pomares T, Martin M, Clarempuy C, Lemezo C, Saint-Marc C, et al. Can we explain environmental contamination by particular traits associated with patients? J Hosp Infect [Internet]. 2019 Dec 20 [cited 2020 Jan 9]; Available from: http://www.sciencedirect.com/science/article/pii/S0195670119305328
13. Sethi AK, Al-Nassir WN, Nerandzic MM, Bobulsky GS, Donskey CJ. Persistence of skin contamination and environmental shedding of Clostridium difficile during and after treatment of C. difficile infection. Infect Control Hosp Epidemiol. 2010 Jan;31(1):21–7.
14. Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013 Aug;26(4):338–44.
15. Cheng VCC, Chau PH, Lee WM, Ho SKY, Lee DWY, So SYC, et al. Hand-touch contact assessment of high-touch and mutual-touch surfaces among healthcare workers, patients, and visitors. J Hosp Infect. 2015 Jul;90(3):220–5.
16. Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007 Jun;65 Suppl 2:50–4.
17. Dancer SJ. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin Microbiol Rev. 2014 Oct;27(4):665–90.
18. Hassan M, Gonzalez E, Hitchins V, Ilev I. Detecting bacteria contamination on medical device surfaces using an integrated fiber-optic mid-infrared spectroscopy sensing method. Sens Actuators B Chem. 2016 Aug 1; 231:646–54.
19. Tekerekoǧlu MS, Duman Y, Serindağ A, Cuǧlan SS, Kaysadu H, Tunc E, et al. Do mobile phones of patients, companions and visitors carry multidrug-resistant hospital pathogens? Am J Infect Control. 2011 Jun 1;39(5):379–81.
20. Sunkesula VCK, Kundrapu S, Knighton S, Cadnum JL, Donskey CJ. A Randomized Trial to Determine the Impact of an Educational Patient Hand-Hygiene Intervention on Contamination of Hospitalized Patient’s Hands with Healthcare-Associated Pathogens. Infect Control Hosp Epidemiol. 2017;38(5):595–7.
21. Rai H, Saldana C, Gonzalez-Orta MI, Knighton S, Cadnum JL, Donskey CJ. A pilot study to assess the impact of an educational patient hand hygiene intervention on acquisition of colonization with health care–associated pathogens. Am J Infect Control. 2019 Mar 1;47(3):334–6.
Infection Prevention and Advocacy: A Conversation About Appropriations and Policies to Enhance the Profession
This article originally appeared in the December 2023 issue of Healthcare Hygiene magazine.
Jill Holdsworth, MS, CIC, FAPIC, NREMT, CRCST, manager of infection prevention at Emory University Hospital Midtown, and Rich Capparell, director of legislative affairs for the Association for Professionals in Infection Control and Epidemiology (APIC), discuss the importance of infection preventionists (IPs) advocating for their profession on Capitol Hill.
Jill Holdsworth: When we talk about advocacy for infection prevention, I don’t think many IPs know what that means or what the hot topics are. Can you give us a taste of what is most important right now?
Rich Capparell: Currently, Congress is struggling to come to an agreement on FY 2024 funding. The House of Representatives and Senate are very far apart, as House measures are looking to make heavy cuts to non-defense spending. APIC has been focused on preserving funding for key infection prevention and control programs, such as the National Healthcare Safety Network (NHSN) and the Antimicrobial Resistance Surveillance Initiative (ARSI). Additionally, we are working with coalition partners to incentivize individuals to enter the infectious disease fields through the Bio-Preparedness Workforce Pilot Program. Although appropriations are important, APIC is also educating lawmakers about policies that will make patients safer. As we know, there are millions of healthcare-associated infections in nursing homes each year and government reports continue to highlight the need for stronger infection prevention and control programs. To see better outcomes, APIC is pushing Congress to require full-time infection preventionists in nursing homes and for these facilities to provide greater HAI data transparency. Finally, APIC is working with a wide array of partners to help support the antibiotic pipeline. To do this we are supporting the PASTEUR Act, which would establish a subscription-style program to provide federal contracts for a reliable supply of critically needed novel antimicrobials and not rely on the volume of sales like most other drugs on the market.
RC: You recently participated in the APIC Board of Directors Lobby Day. Can you share your experiences with Congressional staff (in-person vs virtual)?
JH: Last year, the APIC board of directors met virtually with the congressional staff, which was my first experience with APIC doing this type of work. It gave me the chance to educate myself more on topics most important to infection prevention on a higher level and being able to speak to it and really feel like I was making a difference for all of us and our patients by telling our congressional offices about these items. This fall, we were able to visit the offices in person, which gave us the chance to have a more in-depth conversation about the topics that was really engaging with the staff members. Each APIC board member had an APIC staff member with them and we presented the topics of interest, answered questions and had also done our homework on who we were meeting with, so we also knew what was important to them. This experience was incredible for me personally, allowing me to continue advocating for what is important in our profession, but also what our patients need most.
JH: I know most IPs are going to think they don’t have time for advocacy with everything else we have on our plates. What are some of the things, big and small in terms of time commitment, that IPs can do to make an impact?
RC: There are many ways for APIC members to get involved choose their level of engagement! The easiest way to stay engaged is via the APIC Action eList. This simple action can educate members on key activities and ways they can get involved in the future. For members interested in contacting legislators that aren’t sure what to say, I recommend the APIC Action Center. This resource has pre-written messages on key issues, that take less than a minute to send. Members looking to get more involved can volunteer to be their chapter’s legislative representatives or they can work with staff to start scheduling a virtual meeting with policymakers. Advocacy at any level helps get our message out, so we appreciate all levels of engagement!
RC: As follow-up to your question, what issue did you find most rewarding to talk about?
JH: I have to say I was surprised that it was easiest for me and most engaging to speak to the nursing home staffing ratios, even though that isn’t the setting I work in. This is something we should all be advocating for, as it does impact us all! I was able to speak to scenarios where I have tried to reach “the IP” at these facilities to communicate results, or receive results, and I couldn’t find anyone (or I got someone different each time I asked). The continuum of care is a very real struggle we will continue to deal with until we can ensure appropriate staffing in all facilities where infection prevention resources are needed.
Supplemental Disinfection Technology Use in Long Term-Care Facilities: How, When and Why
By Amanda Sivek, PhD, a-IPC
This article originally appeared in the November 2023 issue of Healthcare Hygiene magazine.
Editor’s note: This article is a summary of “UV Light and Hydrogen Peroxide Vapor Disinfection in LTC Settings: What You Need to Know” co-presented at the 2023 Kairos Education Conference & Exhibit with James Davis, manager of infection prevention and control services at ECRI.
Before the COVID pandemic, the Centers for Disease Control and Prevention CDC) estimated that 1 million to 3 million residents of long-term care facilities experienced a serious infection every year. Infection prevention and control (IP&C) practices can help vulnerable residents avoid getting infections from healthcare workers, other residents, and visitors. Environmental IP&C practices address cleaning and disinfection of environmental surfaces within residents’ rooms and day/common rooms.
At ECRI, we receive many questions from our members about supplemental disinfection technologies like ultraviolet (UV) light and hydrogen peroxide vapor (HPV) devices that are used in a variety of healthcare settings. Let’s discuss how UV light and HPV disinfection work, when these technologies could be used in long term care, and considerations for safely using UV and HPV devices in long term care facilities, such as assisted living facilities, nursing homes, and skilled nursing facilities.
How does UV light and HPV disinfection work?
UV-C light (200-280 nm) and UV-B light (280-315 nm) damage DNA/RNA to prohibit replication of microorganisms, ultimately killing them. UV-C light does not occur naturally on the Earth and is a known carcinogen. UV light disinfection effectiveness depends on the target surface’s distance from the UV light source, shadowing of the surface, and soil load on the target surface. UV light devices used in long term care facilities include UV room disinfection devices, air disinfection devices, UV mobile device disinfection boxes, handheld UV wands and UV phone boxes.
HPV decontamination devices use highly concentrated chemical sterilants to create a pure gas form of hydrogen peroxide that fills an enclosed, unoccupied space. HPV can decontaminate porous and nonporous surfaces within the treated space. A type of HPV decontamination device used in long term care facilities is a portable device with five components: a controller that initiates and tracks HPV device cycles; a dehumidifier that maintains the relative humidity within range to sustain HPV; a vaporizer that creates and disperses HPV with a measured amount of hydrogen peroxide; an aerator unit that breaks down HPV into water vapor and oxygen after the hydrogen peroxide contact time; and at least one hydrogen peroxide sensor that measures HPV concentration within an enclosed area and around sealed outer doors.
When could UV and HPV devices be used in long-term care facilities?
UV and HPV are supplemental disinfection modalities. They can be used after manual cleaning and disinfection methods are performed as shown in the following schematic:
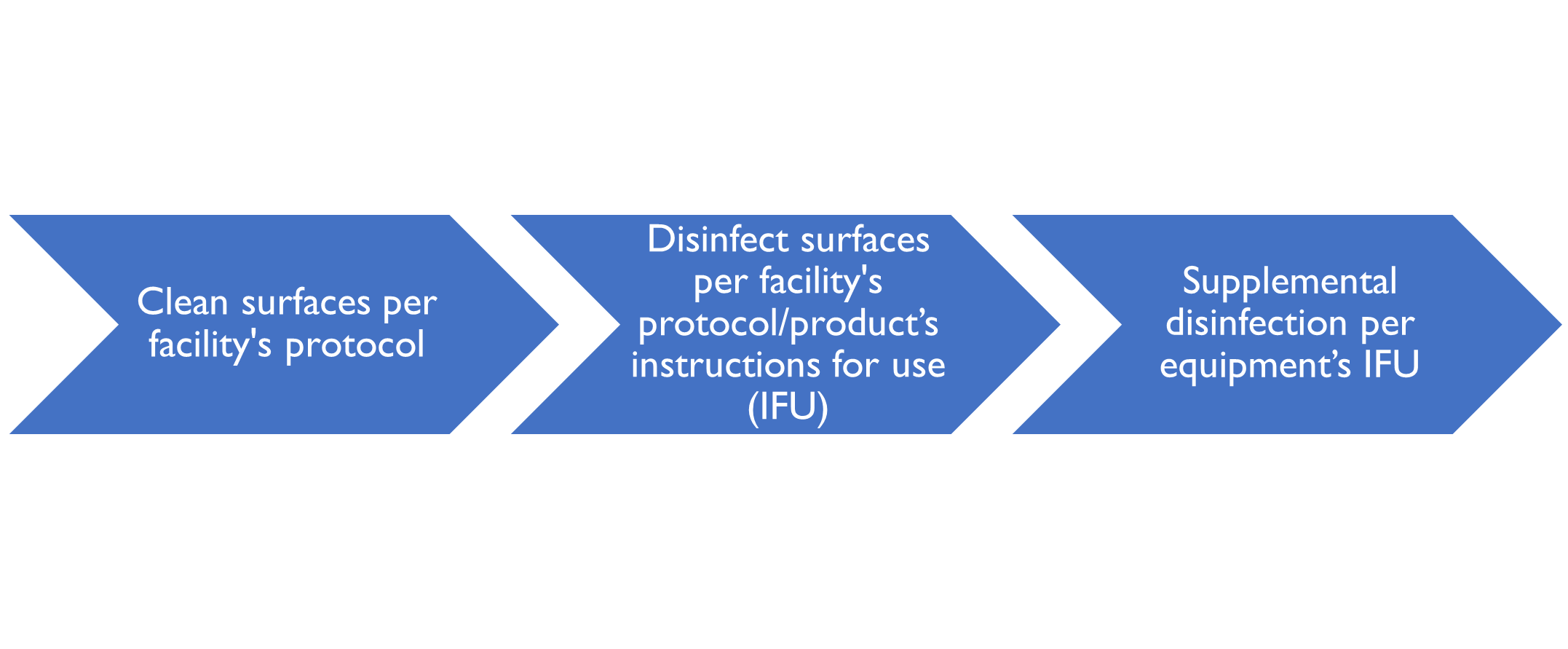
Before using a supplemental disinfection method in a long-term care facility, the first step is to vacate the resident’s room, day room, or resident common area. It is imperative for staff and residents to avoid exposure to UV light and HPV. UV-C light is a carcinogen. Hydrogen peroxide vapor may cause asphyxiation in enclosed areas; other effects from inhalation may include gas embolism, unconsciousness, and respiratory arrest. Bottom line: Stay out of the room when supplemental disinfection devices are in use.
Typical UV Room Device Workflow
After vacating the room, the typical workflow for using a UV room disinfection device is to manually clean and disinfect surfaces within the space, following product IFUs. Next, the room is prepared by placing any safety sensors and the UV device within the room in the designated spots per facility protocol. Finally, the user selects the appropriate cycle, leaves the room, and initiates the disinfection cycle. After the 10- to 40-minute cycle completes, the user reenters the room and moves the device to the resident’s bathroom, another spot in the day/common room, or removes the device from the room.
Typical HPV Device Workflow
Before using a HPV decontamination device in a vacant, enclosed room, users must perform manual cleaning, disinfection, and drying of surfaces within the room, following product IFUs. Next, users should follow the HPV device IFU, which generally indicate to place the portable device in the room; seal HVAC vents, cover smoke detectors, and open cabinets/drawers in the room; place at least one chemical and biological indicator in the room; exit the room; seal all doors to the room using tape; and place a hazard sign at the sealed room doors.
Once the room is prepared, the user initiates a HPV cycle on the device controller and uses the handheld hydrogen peroxide sensor to verify that there is no HPV leakage around the outer doors. Several hours later after the HPV cycle completes, the user returns, unseals the room doors and cracks one door open. They hold the handheld hydrogen peroxide sensor within the room to verify that the chemical concentration is less than one part per million prior to room entry. Once verified, they unseal HVAC vents, uncover smoke detectors, and close cabinets/drawers within the room. Finally, the user documents the results of chemical and biological monitoring. If needed, the user responds to failed chemical and biological indicators per facility protocol.
Why use UV and HPV devices in long-term care facilities?
Supplemental disinfection technologies should only be used safely. Facilities should provide written protocols for each room that UV and HPV devices will be used, a list of any sensitive equipment that must be removed from the rooms, user training on the protocols, personal protective equipment that users should wear, and how users should respond to accidental UV and HPV exposure.
Amanda Sivek, PhD, a-IPC, is principal project engineer II, device evaluation, at ECRI.
ECRI Supplemental Disinfection Technology Resources:
- Evaluation Background: UV Room Disinfection Devices
- Evaluation Background: Countertop UV Disinfection Devices
- Technology Briefing: Hydrogen Peroxide Vapor Room Decontamination Devices
- Hydrogen Peroxide Room Disinfection for Preventing Healthcare-associated Infections
- Dry Hydrogen Peroxide Disinfection Systems for Reducing Healthcare-associated Infections
- Hasty Deployment of UV Disinfection Devices Can Reduce Effectiveness and Increase Exposure Risks(Hazard #6—Top 10 Health Technology Hazards for 2021)
- Avoiding Misuse of UVC Room Disinfection Technology
- Considerations for Clinical Use of Countertop UV Disinfection Devices
- Technology Briefing: Chemical Fog Room Disinfection Devices
- Technology Briefing: Electrostatically Augmented Disinfectant Spray Devices
- Technology Briefing: Far-UVC Disinfection Devices
- Technology Briefing: Filtration and Germicidal UV Light for HVAC Applications
- Technology Briefing: Handheld UV Disinfection Devices
- Technology Briefing: Permanent Environmental UV Disinfection Fixtures
- Technology Briefing: Portable Air Cleaners and UV Air Purifiers
- Technology Briefing: Upper-Air UV Disinfection Devices
- Technology Briefing: UV Mobile Device Disinfection Boxes
- Technology Briefing: UV Phone Disinfection Boxes
- Technology Briefing: UV Room Disinfection Devices
- Technology Briefing: UV Shoe Sole Disinfection Devices
July 1: What is the Significance in Infection Prevention?
By Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST
This article originally appeared in the September 2023 issue of Healthcare Hygiene magazine.
Every year, July 1 comes around and, if you work in healthcare, you either love it or you dread it. Why? A new class of residents and fellows start in our facilities, and the trained, experienced physician trainees leave. There will always be those who feel July 1 means more medical errors and mistakes with less-experienced trainees; however, I have learned to take a different perspective—this is an incredibly exciting time where we can catch this new class when they first come in, teach them good habits right out of the gate so they will teach other residents that come after them correctly, thus creating a cascade effect of proper technique and practice. July 1 should be viewed as a significant opportunity in all areas of healthcare -- especially infection prevention!
It’s easier to dread July 1 than to see the silver lining, but why not try? Having new, bright minds ready to soak up every ounce of training and knowledge they can is such a powerful time. Residents want to do the right thing and they absolutely want to be taught correctly. When asked, many senior residents will tell you they were taught certain skills by the resident prior to them—thus the need to ensure all residents and fellows are taught basic infection prevention skills and knowledge correctly. How does this fit in with infection prevention? There are many ways infection preventionists (IPs) can impact how residents are learning, and thus help prevent infections.
One of my favorite memories of working with our teaching Head & Neck surgical team is when a resident came up to me before a case started in the operating room (OR) and held up a chemical indicator from a surgical drill tray that he had opened to prepare for the case and said, “I am not comfortable with how this indicator turned. I am going to send it back to the sterile processing department (SPD).” This was an incredible moment for our entire team and showed that we had come full circle in what we had talked about, learned and now were able to put it into action to keep our patients safe. But how did we get here?
You must put the time in as an IP — “Go to the Gemba,” as they say. This means to go where the work is done. When it comes to surgical teams, this may look different than your typical rounding with your nursing units, checking for isolation compliance and hand hygiene. To educate surgical teams, you first must understand their workflow, and to do this you must live it. I am the first to admit that this is not easy, and it means getting up very early and spending a lot of time with the team simply observing and learning. The first step is always spending time with the teams you are working with, getting to know their work, their barriers, and their work. When you gain their trust, they will learn from you as much as you learn from them.
When does the surgical team round? Who does the rounding? Who does the surgical prep in the OR? What is the resident’s role in the OR? Who marks the patients for clipping in pre-op? Do residents see the patients in pre-op? These questions will get you started with where you can make an impact with surgical site infection (SSI) protocols with your surgical team. In the past, we have surveyed a service line’s residents at the beginning of the year for general knowledge in skin-prep application techniques such as dry time, application time, as well as sterile processing practices such as checking blue wrap for holes, verifying sterility on chemical indicators, and asking questions about who taught them this information in the past. We spent the next year of their training teaching and emphasizing proper technique and protocols and then we surveyed them again at the end of the year and saw a significant difference in overall attitude and knowledge toward infection prevention processes.
When I spent time educating the surgical residents, I followed up on observing their cases alongside them, being in pre-op with them and tagging along during surgical rounds in the morning. Essentially, I became part of their team too. As an IP, we can either be a teammate or an auditor. I guarantee you that a teammate will get father every time. When I observe cases and attend surgical rounds, I don’t bring a clipboard or even a notebook. I put my hands up and let them know I am here to learn, listen and observe.
Teaching services also will have education sessions during their week—find when these sessions are and put yourself on the schedule. You can emphasize the educational topics you need to cover, show pictures of things you saw during rounds, ask questions about how you can help them, etc. When you become part of the solution, you become a teammate, and everyone begins working together. Everyone in the operating room should know how to check for sterility of instruments, perform an appropriate skin prep, and check an instrument wrap for a hole. When we partner with our physician trainees who may be doing these tasks, we can ensure not only that they are doing these tasks correctly and understand the importance, but that they pass down the correct information to the next class of trainees.
Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST, is manager of the Infection Prevention Department at Emory University Hospital Midtown in Atlanta.
Rationing Rather Than Omitting Care: A Nursing Expert Addresses an Alarming Trend
By Kelly M. Pyrek
This article originally appeared in the August 2023 issue of Healthcare Hygiene magazine.
In the February 2023 and April 2023 issues of Healthcare Hygiene magazine, we examined the impact of missed nursing care on patient safety as well as infection prevention and control. In this column, we feature a conversation with nursing expert Kasia Bail, PhD, a professor of gerontological nursing at the University of Canberra in Australia, about the tough choices nurses make during every shift.

Kasia Bail, PhD
HHM: What do you believe has been the impetus for the recent number of papers on missing care?
Kasia Bail: Given the time that it takes to establish research and publish it, I suspect that the surge in numbers has more to do with the prevalence of the issue and the momentum that science gains when there’s a phenomenon that justifies examination. Developing and examining evidence is slow and happens incrementally and has been building for the last 20 years.
HHM: Did COVID perhaps bring this issue to the forefront? And how might COVID have exacerbated an already problematic trend of missed nursing care?
KB: This has certainly been the case and there are publications to support it (See: https://qualitysafety.bmj.com/content/30/8/639.abstract). I would argue that “missed nursing care” isn’t a new problem so much as it is a labeling of an ongoing issue. Nursing will always, and has always, rationed care in different ways. I think we need to involve the public in choosing what it wants nursing to ration. For example, my older nursing peers will often make jokes about how they used their time during shifts to make sure none of the pillowcase openings were facing the door, and to line up the wheels on the bed. Anecdotally, this was clinically and theoretically practical, to make sure the sand didn’t get into the pillowcases and the beds could be wheeled into the operating theatre promptly – various hang-ups from the Crimean War, perhaps. There is no evidence that I’m aware of, but one can assume nurses were choosing to use their time on these activities, rather than perhaps talking with patients or supporting family relationships or performing hygiene practices, which might have a stronger evidence base and cultural support now. Pillowcases and trolley wheels might sound like facetious examples, but the point being that the activities that nurses spend time on have always required prioritization; care has always been rationed. These days, if a nurse must choose between brushing someone’s teeth, administering a life-saving antibiotic on time, and admitting a new patient onto the ward so that a new bed can be created in the emergency department, it is logical that the teeth brushing will be the lowest priority. That doesn’t mean a nurse chooses not to do it but chooses not to do it right now and may choose to delay it within her shift or to hand it over to the next nurse. In the “Failure to Maintain” article I referred to, this kind of nurse decision making as “in-hospital triage.” I am a strong advocate for a “nurse interrupted” button in new digital information systems. Nurses work in multi-tasking, interrupted manners, yet many of the workflows being developed don’t recognize this and try to tie the nurse to the computer to complete her documentation. Nurses need to be able to make these decisions about immediate prioritization and be supported in delaying or postponing care but still communicating their need, to create a functional work environment that recognizes the reality of care rationing. Similarly, we need better data to help us make these decisions, and to support resourcing. For example, we often don’t have any clear measurable and comparable indicators about patient load – whether patients are self-caring and independent with showers or need bed sponges and two-hour wound dressings. Nurses are the flex of any hospital system, and we rely on their decision making within patient allocations and ward allocations to ensure care continues to be delivered. But that is rarely acknowledged in hospital governance systems, and minimal research. I recommend a key measure is the introduction of International Functional Standard – currently in use in rehabilitation hospitals only – to be included in acute-care hospitals for ICF (International Classification of Functioning, Disability and Health). This would still be a crude measure, but it would provide some indication of workload. There are many in use across the world, but many are resource intensive and require nurses to do more work to identify how much work they must do, which is ironic.
HHM: Is the current focus on healthcare professional burnout and nurse exodus from the field peri- and post-pandemic perhaps shedding more light on the issue of missed nursing care?
KB: The professional burnout has been an ongoing issue, but not really addressed, hence the ongoing issues. The pandemic brought the underlying issues to the fore at a time when even the best health systems in the world are stretched. The National Health Service (NHS) reaching its 75th birthday has been highlighted about the conflation of expectations and health management strategies (See: https://www.theguardian.com/books/2023/jun/17/fighting-for-life-by-isabel-hardman-our-nhs-by-andrew-seaton-review-the-nhs-at-75). The intersection between burnout and missed nursing care, and the factors that can ameliorate them, is a key area for research and intervention. The new attempt at Magnet rollout in Europe attests to this. (See: https://www.magnet4europe.eu/ and https://www.magnet4europe.eu/blog-page/four-questions-to-walter-sermeus)
HHM: Is the average nurse even aware of the concept of Cascade iatrogenesis? Is it a concept that should be included in nursing education?
KB: That’s a good question. Certainly, all the undergraduates in my university course would be exposed to this concept; however, university degrees are often contested with so many specialty areas vying for space. I suspect that as a complex issue it is perhaps not well covered (it would be a great research project for an honors or PhD student). Many health organizations don’t understand it well; all over the world we are trying to get the right balance of risk assessment (sometimes called comprehensive assessment), and then providing ameliorating or risk-modifying interventions. However, these are in addition to whatever the admitting diagnosis is (for hospitals, or other health services similarly). I would argue that cascade iatrogenesis is less the issue, than the ability of the staff to provide comprehensive care. In other research I’ve done it’s shown that nurses are under such pressure to complete the assessments – that is what is audited – but what care they provide to respond to that assessment is harder to audit. And so, there’s a perverse incentive, based on how clinical governance ends up working, that emphasizes the assessment and its documentation rather than the delivery of care. The reason this is an issue in relation to cascade iatrogenesis is that health services struggle to get the right balance to support staff to make decisions about patients that may be deteriorating. Missing someone’s cup of tea is a justifiable decision when it means making sure someone else’s antibiotic is administered on time. But when multiple cups of tea get missed, and then dehydration occurs, and then that isn’t assessed or identified and responded to, then the risk profile for that patient goes up. But arguably a large issue as to why cascade iatrogenesis may be occurring is less about what is taught, as what is translated into practice, and what is sustained based on the work environments. We also need to promote that the higher risk of complications means the need for higher prophylaxis. That sounds logical – when the risk for deep vein thrombosis is higher, there are protocols for clexane or heparin; when the risk for infection is higher, then there are protocols for antibiotic cover. However, with many of the competing nursing issues – confused patients, long wound dressings, complex medication regimes – there aren’t protocols to increase the prophylaxis, because the increased prophylaxis is increased nursing care. We have no clear way to “increase the nursing dose.” We rely on clever and articular shift managers, on ward managers communicating their needs to hospital administrators, or reviews of workload models, sometimes in conjunction with nursing unions. So, I would reiterate – we need to promote that higher risk of complications means the need for higher prophylaxis, and nursing care needs to be recognized and recommended as prophylaxis.
HHM: Do most nurses struggle with their decision to omit care, or is it more of an unconscious occurrence?
KB: This is a difficult question to answer. The old adage about “applying the oxygen mask to yourself before helping others” must be considered. Self-preservation is a natural response under threat – the increased pressure during covid that has intensified the front-line responses as well as public awareness of these tensions. Yes, nurses struggle with any decisions to omit care, but significantly, I would argue for the language of “rationing” rather than “omitting care.” All healthcare gets rationed, all health services make decisions about what they can and can’t provide, who’s within and who’s outside the boundaries of care, which medications make it to the subsidized list, which services are provided to which areas, which rural area gets the next diagnostic scanning machine (X-ray, MRI, CT, PET). But the difference with nurses is that it’s very direct, it is within the four walls of the ward that the decisions are made, which is all the more reason that the emotional labor of those nurses making the decisions must be considered. Research shows that many nurses leave roles when they are dissatisfied with the quality of care they are able to provide. But equally, yes, nurses will unconsciously omit care. There is research that highlights that some rationing of care can become habitual – that nurses may be used to being busy and get used to using the most streamlined approach they have developed to save them in times of duress. I believe patient teeth-brushing has been habitually sacrificed, and the evidence seems to back this, as one of the most commonly rationed nursing tasks. But my hospital used to have backup toothbrushes and toothpastes but no longer does; we hardly seem to have any kidney dishes and most of the patients are bedbound so without a kidney dish it’s hard to help them brush their teeth in bed. And not all wards stock the mouth buds, which quite frankly are fine for a quick mouth refresh for a dying patient but not particularly useful if someone needs a vigorous toothbrushing. So, if there isn’t the environmental nudge toward this good practice then it can reinforce this habitual de-prioritization. Will the after-hours shift coordinator prioritize delivery of a toothbrush at 9pm? I doubt it (I’m happy to be corrected!), but they would an antibiotic, so that helps you see what practices get enabled and prioritized systemwide. I think that increasingly missed nursing care is also being taught in the nursing curricula, particularly given the increasing science supporting and investigating the phenomena. I think the bigger issue is the shock that people experience about nurses having to ration their care. There will always be a finite number of resources. Health is no different to other areas of the economy, but arguably is dealing with the conflating issues of ageing populations, which means an increased volume of the population with complex illnesses that require ongoing treatment (rather than dying from their conditions); as well as increased expectations about health and medical care due to increased technology and specialization in responding to conditions, as well as increased transparency in terms of clinical governance. These do great things to support quality care, but they also put additional pressure on those settings to deliver. Nurses will always be the face of many of these health interactions, and in hospital settings in particular, the tensions between who receives the resource – in this case, nursing time -- is more visible.
HHM: In your study, were the nurses working as general floor/unit nurses or were they specifically tasked with infection control-related duties? Did this make any difference in their behavior?
KB: Seven of the 11 interviewees were directly in infection control nursing roles. The other four had varied roles which are specified. Further research, including quantitative research which seeks to differentiate different types of nurses and their value of infection control practices, would be interesting. I think this article highlights, however, that staffing needs to be controlled for the questions of “Do higher proportions of RNs affect the value placed on infection control? Or does a higher proportion of RNs reduce the incidence of missed nursing care?” Missed nursing care research is very hard to do prospectively however, hence the dearth of research in this arena.
HHM: Is missed care attributable to a gap in knowledge or a gap in practice, or both, and should this be a wake-up call that training and education need improvement?
KB: I think that is an oversimplification of complex issues, which is all too common in the interpretation of nursing work. I would encourage a review of the sections in our paper on the “whole of hospital” approach on pages 4-5. These nurses talked about how they needed to be able to talk convincingly to get the support of accountants to make appropriate purchases. The nurses also talked about there being excellent policies in place, but not enough nurses to make the policies deliverable. These are issues beyond teaching undergraduate nurses, or a gap in translating their learnings into practice – it’s a wake-up call that all layers of hospital administrators need to better understand and respect infection control nursing work, collect local data, and respond meaningfully.
HHM: Is it simply a matter of resourcing and staffing, to address missed nursing care in infection control-related practices, or is it also a behavioral issue/human factors engineering-related issue?
KB: It’s absolutely both. We know from the magnet hospital research, which has continued to be supported through decades of research (and increasing continental reach, with the European Magnet Hospital program in mid-delivery) that hospitals with good work environments have better outcomes. We know that the ingredients of these healthy work environments include resourcing and staffing as well as sound clinical governance and trusting and effective relationships between nurses, managers and other health professionals.
HHM: You make a very key statement in your paper when you observe “There are many situations of clinical care delivery that have not had robust research conducted to support the practice, despite widespread expert recommendations." This is a very big reason why many nurses in the U.S. believe they can skip some infection prevention-related practices, so how can this be addressed by health systems?
KB: Great question. Healthcare is always delivered in a state of uncertainty – evidence is continually being developed and refined, and some elements of care will always have a more theoretical principled approach and will not be able to develop a gold standard prospective clinical trial to “prove” an association or demonstrate the ‘best’ way. There’s an interesting article on the World Health Organization’s 5 Moments of Hand Hygiene highlighting the impracticality of this “protocol” which doesn’t have an evidence base. That doesn’t mean it doesn’t work – it is a very sound theoretical approach, and as an adoptable system has likely increased the hand hygiene practices and therefore infection rates. (See: https://qualitysafety.bmj.com/content/31/4/322 and https://www.sciencedirect.com/science/article/pii/S0195670123000725) Clinicians are accustomed to working in very messy settings where the group norms determine the practices, as well as making their own clinical decisions – that’s what they are paid to do, as registered health professionals. This is why some data and monitoring, as well as healthy working relationships, are crucial to be able to assess and interpret practices in an ongoing manner. Again, the magnet hospital approach supports this transparency and openness to practice development and change. (See: https://www.nursingworld.org/organizational-programs/magnet/magnet-model/ and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4431919/)
HHM: This is also a key observation from your paper: "Activities are prioritized in relation to the perceived impact of non-performance" -- are nurses taking it upon themselves to reject evidence-based practice because they think there is negligible impact on patients? Isn't this a potential undoing of any patient safety-first nursing care?
KB: I think that is an extreme interpretation, regarding “rejecting evidence-based practice.” The point of evidence-based practice is for the clinician to make interpretations of the evidence, and apply them in relation to the current situation, and their knowledge of the patient. I often tell my students to imagine they have one hand on the patient, one hand on their pile of textbooks and journals, and they themselves are the body in the middle with the eyes and ears to interpret the situation and try to make the best possible decision. I would argue that nurses work with a never-ending litany of contextually imagined yellow and red flags of possible future, potentially likely and actual and immediate threats to patient safety on a minute-by-minute basis. They manage this through “cognitive stacking” – continually re-prioritizing their mental list of tasks and worries as their shifts progress (See: https://journals.lww.com/jonajournal/Abstract/2005/07000/Understanding_the_Cognitive_Work_of_Nursing_in_the.4.aspx). It is exhausting, exacting and delicate work. They use their knowledge of their patients, biology, pathophysiology, pharmacology, hospital systems, hospital policies, hospital personalities and time and other resources to make these decisions on a minute-by-minute basis. An activity that will alleviate an immediate and actual threat to safety (and, of course, patient comfort) may need to take priority to a possible threat with a lower likelihood of risk outcome. It is a never-ending and constantly adapting risk matrix that the nurse conducts without much support. It has been argued that nurses would benefit from “clinical supervision” – the approach that psychologists use to reflect on the complexities of their role in working patients and use a mentor to help reflect on decision making and allow space for growth and adaptation. This is being taken up in a number of jurisdictions. This reflective practice would offer an opportunity to review practices and policies with a peer. Arguably this used to often happen in handovers, however handovers are now often done by tape recorder or at the bedside and/or with the computer, potentially limiting the reflecting learning space that they provided. In the style of magazine questionnaires, I have compiled some examples of the kinds of impossible decisions nurses deal with on a minute-by-minute basis:
- You have 10 minutes left in your shift. Do you:
- Check whether Mary’s pain relief has worked or if she needs another 5mg of endone.
- Check the emergency trolley and make sure all equipment is present and sterile if appropriate in case of a life-threatening code next shift.
- Go home early. You’ve already done two hours unpaid overtime this week.
- You are interrupted mid-task. Do you:
- Continue your task of educating Mr. Aliia regarding his colostomy bag, including infection control practices and emotional support for coming to terms with his new body
- Stop your current task, and attend to Mavis the patient in the next room who you have been told is nauseous and about to vomit
- Depends on whether you like Mr. Aliia or Mavis better
- You are set up to do a catheter insertion on Maria. You realize you may have touched the tip of the sterile catheter with your sterile glove but you’re not sure. Do you:
- Stop the procedure, and set it all up again, because the risk of infection is present and you would like to save Maria from that risk
- Continue the procedure, because you’re not certain you did touch it so the risk is small, and also because Maria is confused with delirium and also doesn’t speak English as a first language, so you think you would do more harm by lengthening the procedure, and also because relieving her distended bladder with the catheter may ease her delirium, which is a higher and more immediate risk than the risk of infection
- Call out to see if another nurse can help you make the decision
Reference: Bail K, et al. Missed infection control care and healthcare-associated infections: A qualitative study. Collegian. Vol. 28, Issue 4. Pages 393-399. August 2021.
Navigating the Environmental Protection Agency’s Lists of Disinfectants
By Katherine Lunt, MPH, MBA, CIC, HEM
This article originally appeared in the July 2023 issue of Healthcare Hygiene magazine.
In healthcare, cleaning and disinfection are extremely important to prevent the spread of infections. Cleaning is the physical removal of visible dirt, blood, body fluids, and other foreign material from objects and surfaces and must be performed prior to disinfection. Disinfection is the process of destroying microorganisms, such as bacteria, viruses, and fungi. There are three levels to categorize disinfectants: low-level, intermediate-level, and high-level disinfectants.
High-level disinfectants are intended to be used for critical and semi-critical medical devices and instruments; high-level disinfectants are regulated exclusively by the Food and Drug Administration (FDA) and are not intended to be used on environmental surfaces. Intermediate-level disinfectants are intended to be used on some semi-critical items and non-critical items. Low-level disinfectants, such as environmental surface chemical disinfectants, are intended to be used on noncritical items. Intermediate-level and low-level disinfectants are regulated by the Environmental Protection Agency (EPA) and have EPA registration numbers.
The Environmental Protection Agency (EPA) and Chemical Disinfectants
There are several types of disinfection methods used in healthcare. The most common method of environmental disinfection is chemical disinfection. Chemical disinfection is a process that uses chemicals to destroy microorganisms. Some common chemical disinfectants for environmental surface disinfection used in healthcare include alcohols, quaternary ammonium compounds, phenolics, and sodium hypochlorite (i.e., bleach). In the United States, the EPA regulates pesticides, including chemical disinfectants, used in healthcare to ensure they are safe and effective. An EPA-registered chemical disinfectant has been evaluated by the agency and certifies that the disinfectant is effective against the pathogens specified on the disinfectant’s label. Appendix A outlines the lists of antimicrobial products registered by the EPA.
The EPA requires laboratory potency testing for products to support product label claims. Chemical disinfectants labeled as “hospital disinfectant” have passed potency testing for activity against three representative organisms: Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella cholera suis. Hospital disinfectants that demonstrate potency against mycobacteria may include “tuberculocidal” on the label as well and are considered an intermediate-level disinfectant. A chemical disinfectant labeled as a hospital disinfectant without a tuberculocidal claim is considered a low-level disinfectant.
Selecting a Disinfectant on an EPA-Registered Disinfectant List
Chemical disinfectants are often marketed and sold under different brands and product names. To find out more information about a specific chemical disinfectant, locate the EPA-registration number on the product label. The EPA-registration number is listed on the product as EPA Reg. No. and is followed by two or three sets of numbers on the label. Search the EPA-registration number in the specific EPA-Registered Disinfectant List of the pathogen you are trying to kill exactly as the number appears on the label. If the disinfectant is EPA-certified, it will populate on the list and include the following information:
The Registration number is listed on the product. This is to help you identify the product on the EPA disinfectant lists.The Active Ingredient/s is the ingredient in the disinfectant that destroys the pathogen.
The Product Name is the common name of the product.
The Company is the manufacturer of the disinfectant.
The Contact Time in Minutes (“dwell time” or “wet time”) is the amount of time that the surface must remain wet for the disinfectant to work effectively.
The Formulation Type denotes whether the disinfectant is ready-to-use or requires a dilution for safe use.
The Surface Types describes the type of surfaces that this disinfectant can be used on. For example, many disinfectants can only be used on hard, nonporous surfaces, which would include most high-touch surfaces.
The Use Sites (Hospital, Institutional, Residential) are the settings where the product is intended to be used.
If there is not an EPA-registered disinfectant list available for a specific multidrug resistant organism (MDRO), search for a product claim against the organism or bacteria itself. To is important to highlight that MDRO denotes that the organism is resistant to treatment, not disinfection. MDROs are still susceptible to disinfection. Occasionally, a disinfectant will include kill claims against a specific MDRO. If the chemical disinfectant is effective against a drug-resistant form of the organism, it is effective against all forms of the organism.
Always follow the information on the product’s label and adhere to the instructions for use (IFU), personal protection equipment (PPE) requirements, and contact time to ensure maximum efficacy of the chemical disinfectant.
Appendix A – Antimicrobial Products Registered with EPA for Claims Against Common Pathogens
List A: Antimicrobial Products Registered with the EPA as Sterilizers
List B: Antimicrobial Products Registered with EPA for Claims Against Mycobacterium tuberculosis (TB)
List C: EPA’s Registered Antimicrobial Products Effective Against Human HIV-1 Virus
List D: EPA’s Registered Antimicrobial Products Effective Against Human HIV-1 and Hepatitis B Virus
List E: EPA’s Registered Antimicrobial Products Effective Against Mycobacterium tuberculosis, Human HIV-1, and Hepatitis B Virus
List F: EPA’s Registered Antimicrobial Products Effective Against Hepatitis C Virus
List G: Antimicrobial Products Registered with EPA for Claims Against Norovirus (Feline calicivirus)
List H: EPA's Registered Antimicrobial Products Effective Against Methicillin-Resistant Staphylococcus aureus (MRSA) and/or Vancomycin-Resistant Enterococcus faecalis or faecium (VRE)
List J: EPA’s Registered Antimicrobial Products for Medical Waste Treatment
List K: Antimicrobial Products Registered with EPA for Claims Against Clostridium difficile Spores
List L: Disinfectants for Use Against Ebola Virus
List M: Registered Antimicrobial Products with Label Claims for Avian Influenza
List N: Disinfectants for Use Against SARS-CoV-2
List O: Disinfectants for Use Against Rabbit Hemorrhagic Disease Virus (RHDV2)
List P: Antimicrobial Products Registered with EPA for Claims Against Candida Auris
List Q: Disinfectants for Emerging Viral Pathogens (EVPs)
Katherine Lunt, MPH, MBA, CIC, HEM, is an infection preventionist with ECRI.
References:
CDC. 2019. Background E. Environmental Surfaces. https://www.cdc.gov/infectioncontrol/guidelines/environmental/background/services.html.
CDC. 2016. Chemical Disinfectants. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html.
CDC. 2016. Cleaning. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/cleaning.html.
CDC. 2016. Guideline for Disinfection and Sterilization in Healthcare Facilities (2008), Tables and Figure: Table 1. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/tables/table1.html
Environmental Protection Agency (EPA). 2022. Selected EPA-Registered Disinfectants. https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants
WHO. 2018. Table 3.3.3, Spaulding Classification of Equipment Decontamination. In: Global Guidelines for the Prevention of Surgical Site Infection. https://www.ncbi.nlm.nih.gov/books/NBK536426/table/ch3.tab7/
Biofilms and Healthcare
By David W. Koenig, PhD
This article originally appeared in the June 2023 issue of Healthcare Hygiene magazine.
Healthcare facilities -- such as hospitals, nursing homes and outpatient facilities -- are opportunistic locations for acquiring secondary infections unrelated to a patient's primary condition. Healthcare-acquired infections (HAIs) are a primary concern for healthcare providers, administrators, and governments worldwide due to the reduced quality of healthcare and the considerable associated socioeconomic costs resulting from extended hospital stays for infection treatment.
Some of the most common HAI types occur during the use of indwelling medical devices, such as a catheter, endotracheal tube, feeding tube or prosthesis. According to the Centers for Disease Control and Prevention (CDC), the most common underlying cause of infections related to in-dwelling medical devices is the ability of microorganisms to adhere to the surface of devices and form biofilms. Biofilms are often associated with tissue infections such as chronic wounds, skin infections, endocarditis, chronic otitis media and cystic fibrosis.
Biofilms are a gathering of microbes that colonize various surfaces. Many biofilms are beneficial. The beneficial human microbiome consists of diverse biofilms on the skin, teeth and mucosa of the gut, nasal, and reproductive organs; however, if a biofilm contains pathogens, these biofilms can become a severe concern for healthcare facilities, leading to HAIs.
The aspect of most concern is increased resistance to antimicrobials and antibiotic therapy; because of this concern's broad acceptance that the most effective way to reduce the incidence of medical device-related infections is to prevent primary microbial adhesion and subsequent biofilm formation.
Biofilms produce an extracellular polymeric substance (EPS) that protects the microbes in the biofilm. EPS interferes with the penetration of antibiotics or antimicrobials through biofilm. EPS interference is compounded by the cells in the biofilm having an altered physiology that further protects those cells from any antimicrobial that might penetrate the biofilm. Biofilms also provide the microbes with an environment that allows cell-cell communication and quorum sensing and enhances the transfer of genetic elements and resistance genes. Indeed, biofilm bacteria can transform into a persistent state that mimics a spore. Ultimately, biofilms increase the prevalence of antibiotic-resistant microbes and the risk of transmission to the caretaker and patient.
Healthcare environmental biofilms can act as reservoirs for the transmission of pathogens. Biofilms are commonly associated with surfaces that remain wet; however, a biofilm cover surface does not necessarily have to appear watery to the eye for a biofilm to persist. Recently, dry surface biofilms have been receiving extensive study. Dry surface biofilms form an exterior veneer while the surface is wet and then dry when the moisture dissipates. Biofilm EPS plays a critical role in the persistence of a dry biofilm allowing the retention of enough water for sustained survival. Biofilm "hot spots" can include drains, sinks, plumbing connections, areas of toilets that remain wet and not cleaned with mechanical action, bathrooms, sink traps, air filtration mediums, window ledges and seals, air conditioning systems, fabrics, and carpets and rugs. Dry surface biofilms grow on various surfaces, such as blood pressure cuffs, intravenous poles, door handles, touch screens, and cell phones. Dispersion of cells from biofilms can perpetuate microbial resistance, recurrence, and transmission of dangerous pathogens in the healthcare environment.
The types of microbes associated with biofilms are very diverse. The most common bacteria associated with hospital device-related infections is Staphylococcus epidermidis. Other hospital biofilm bacteria are methicillin-resistant Staphylococcus aureus (MRSA), Viridans Streptococci, Enterococcus faecalis, Vancomycin-resistant Enterococci (VRE), Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Proteus mirabilis, and Klebsiella pneumoniae. Recently, the fungi Candida auris has emerged as a critical biofilm-associated pathogen. Other medically associated fungi that form biofilms are Aspergillus, Cryptococcus, Trichosporon, Coccidioides, and Pneumocystis— furthermore, human viruses and bacteriophages present in biofilms.
Control of biofilms is complex. For device-related biofilms, various prevention strategies are initiated. One process is to impregnate the device with leachable antimicrobials such as silver. Another is to coat the surface with anti-adherents that interfere with the initial attachment of the microbe to the surface, interfering with the 1st step of biofilm formation. There is also the possibility of imprinting micro-patterns on surfaces that inhibit biofilm formation. These tactics have allowed various levels of protection from biofilm formation on devices. Removing biofilms from animate surfaces commonly involves mechanical methods such as water jets and sonic disruption, widely found in dental cleaning.
Prevention and removal of biofilms on inanimate environmental surfaces are just as challenging. The biofilm is often on a surface that is hard to reach or in a dead leg within a water system. Cleaned and disinfected surfaces contiguous to the contaminated area can be readily re-contaminated by biofilm dispersion leading to a transmission hot spot. A strategy to help reduce surface recontamination is to employ a persistent antimicrobial. For example, copper-containing materials, such as bed rails, have prevented biofilm formation. Using a residual disinfectant may also be an excellent option to reduce the recontamination of a surface.
Biofilms are inherently resistant to chemical disinfectants; therefore, mechanical and chemical methods are usually employed to treat environmental biofilms effectively. Mechanical methods may include abrasive scrubbing, high-power sprays and jets, and sonic cleaning, to name a few. Strong oxidants such as peracetic acid are good candidates for treating biofilms, although insufficient evidence exists to distinguish between product performance and biofilms. Recently, there has been a trend for disinfectant manufacturers to evaluate disinfectant performance against biofilms. Additionally, if the biofilm matrix or dead biofilm cells remain on a surface after cleaning, biofilms will form faster than surfaces free of the contaminating material. Removal of the biofilm matrix after disruption on surfaces points out the importance of removing biofilm debris as a prophylactic for future biofilm control.
Healthcare biofilm control is a cornerstone in reducing antimicrobial resistance and making a significant dent in HAIs. Everyone involved in cleaning must better understand and grasp the types of disinfectants required to control healthcare biofilms. Therefore, more effort is needed in all these knowledge areas to help healthcare practitioners and caregivers develop efficient tactics to identify, prevent, and remove biofilms.
David W Koenig, PhD, is principal of DKMicrobios LLC, founded in 2022 to provide consulting microbiology and skin biology services for R&D, technology discovery, product development, R&D strategy and portfolio assessment, IP landscaping, technical writing, and EPA submissions. Koenig has 35-plus years of experience in the life sciences (microbiology and skin biology) with government labs (NASA), private industry R&D, and academia. He also is a member of the advisory board for the Environmental Services Optimization Playbook (EvSOP).
References:
Aillón-García P, Parga-Landa B, et al. Effectiveness of copper as a preventive tool in healthcare facilities. A systematic review. Am J Infect Control. 2023.
Alonso VPP, Gonçalves MPM, et al. Dry surface biofilms in the food processing industry: An overview on surface characteristics, adhesion and biofilm formation, detection of biofilms, and dry sanitization methods. Comprehensive Reviews in Food Science and Food Safety, 22(1), pp.688-713. 2023.
Barnes M, Feit C, et al. Antimicrobial polymer modifications to reduce microbial bioburden on endotracheal tubes and ventilator-associated pneumonia. Acta biomaterialia. 91, pp.220-234. 2019.
Brandwein M, Steinberg D, et al. Microbial biofilms and the human skin microbiome. NPJ biofilms and microbiomes, 2(1), p.3. 2016.
Dancer SJ. How Do Biofilms Affect Surface Cleaning in Hospitals? Hygiene. 2(3), pp.132-135. 2022.
De Vos WM. Microbial biofilms and the human intestinal microbiome. NPJ biofilms and microbiomes. 1(1), pp.1-3. 2015.
Fanning S and Mitchell AP. Fungal biofilms. PLoS Pathogens. 8(4), p.e1002585. 2012.
Ferrer MD and Mira A. Oral biofilm architecture at the microbial scale. Trends in microbiology. 24(4), pp.246-248. 2016.
Gu H, Lee SW, et al. Magnetically driven active topography for long-term biofilm control. Nature Communications. 11(1), p.2211. 2020.
Harper DR, Parracho HM, et al. Bacteriophages and biofilms. Antibiotics. 3(3), pp.270-284. 2014.
Horton MV and Nett JE. Candida auris infection and biofilm formation: going beyond the surface. Current clinical microbiology reports. 7, pp.51-56. 2020.
Ikner LA, Rabe AB and Gerba CP. Residual Sanitization of Three Human Respiratory Viruses on a Hard, Non-Porous Surface. bioRxiv, pp.2023-03. 2023.
Iseppi R, Sabia C, et al. Virulence factors, drug resistance and biofilm formation in Pseudomonas species isolated from healthcare water systems. Current Microbiology. 77, pp.1737-1745. 2020.
Jorge P, Magalhães AP. Antimicrobial resistance in three ways: healthcare crisis, major concepts, and the relevance of biofilms. FEMS Microbiology Ecology. 95(8), p.fiz115. 2019.
Mazaheri T, Ripolles-Avila C and Rodríguez-Jerez JJ. Elimination of mature Listeria monocytogenes biofilms formed on preconditioned and non-preconditioned surfaces after the application of cleaning treatments and their cell regeneration. LWT, 173, p.114316. 2023.
Mazaheritehrani E, Sala A, et al. 2014. Human pathogenic viruses are retained in and released by Candida albicans biofilm in vitro. Virus research. 179, pp.153-160.
Moore G, Stevenson D, et al. Biofilm formation in an experimental water distribution system: the contamination of non-touch sensor taps and the implication for healthcare. Biofouling. 31(9-10), pp.677-687. 2015.
Nkemngong CA, Voorn MG, et al. A rapid model for developing dry surface biofilms of Staphylococcus aureus and Pseudomonas aeruginosa for in vitro disinfectant efficacy testing. Antimicrobial Resistance & Infection Control, 9(1), pp.1-9. 2020.
Richardson M and Rautemaa-Richardson R. Exposure to Aspergillus in home and healthcare facilities' water environments: focus on biofilms. Microorganisms. 7(1), p.7. 2019.
Saleem Z, Godman B, et al. Point prevalence surveys of health-care-associated infections: a systematic review. Pathogens and global health. 113(4), pp.191-205. 2019.
Tahir S, Chowdhury D, et al. Transmission of Staphylococcus aureus from dry surface biofilm (DSB) via different types of gloves. Infect Control Hosp Epidemiol. 40(1), pp.60-64. 2019.
Vadyvaloo V and Otto M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. The International Journal of artificial organs. 28(11), pp.1069-1078. 2005.
Yin W, Xu S, et al. Ways to control harmful biofilms: prevention, inhibition, and eradication. Critical reviews in microbiology. 47(1), pp.57-78. 2021.
Zhen X, Lundborg CS, et al. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrobial Resistance & Infection Control. 8, pp.1-23. 2019.
Infection Prevention and Surgeon Collaboration: The Secret Sauce to Working Together
By Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST
This article originally appeared in the May 2023 issue of Healthcare Hygiene magazine.
Preventing surgical site infections (SSIs) can seems very daunting to infection preventionists (IPs) and one of the hardest parts of forming an interdisciplinary team can be finding an engaged surgeon champion who wants to make changes globally for all service lines. Where do you start with surgeon engagement? How do you involve physicians in general to partner for success? I have found a multi-factorial approach is the best way to go when working on any type of healthcare-acquired infection (HAI), but it is even more important when tackling SSIs.
Finding Common Ground: I have heard physicians say that finding something that we both find important to connect with is the key to starting a partnership. In other words, “What’s in it for me?” Most physicians have a to-do list that the infection prevention department can help them with, but they don’t know where to start. Once you begin a partnership with SSI work, you can start to learn what they have on their minds, form action plans and get to work. One personal tip—work on things that are service line specific with your surgeon champions, so the tasks are personalized and feel very tailored to their needs.
Rounding Together: How often do you round with your surgical teams? I have one warning for you—you will need to wake up very early. The rewards far outweigh the lack of sleep. When I started rounding with our ENT/head and neck surgical team, I first had to walk in with my hands in the air and tell them I am honestly here to learn from them, not to audit. As IPs, it’s very hard to round anywhere or with anyone and not be viewed as the “auditor” or “surveyor.” I made it a habit to not bring a notebook or anything to write on to prove I was there to simply observe, learn and see what they did each morning. To be honest, this took some time before we all built trust with each other, but when this happened, the results were more than we could have asked for. They started calling me when they found issues, they invited us to their resident education sessions to discuss certain topics like skin prep, hand hygiene, scope processes, etc. We even found common ground in the operating room, where we improved the pre-op clipping process with our head and neck surgeries, skin prep and understanding how to check sterile indicators in instrumentation—all because the IP team put away their clipboards and were humble enough to learn and observe FIRST and put building relationships first.
Our SSI champion as well as head and neck surgeon, Dr. Brian Boyce, says, “Collaborating with our infection prevention team has been critical to the success in reducing post-operative surgical infections in our patients. Our infection prevention team brings a unique perspective to the entire perioperative process that enabled us to identify and mitigate risk factors for SSI.”
Involving the Residents: As mentioned above, residents can be your most influential asset when learning about the opportunities and challenges or barriers in various aspects of prevention infections.
Surgical Instruments: Show the surgeon. Have you ever gone through the surgical set with the surgeon? Give it a try. Going through each set, inspecting the instrumentation in each set together as a team and using this as a quality tool for the surgical team, infection prevention department and sterile processing department can help everyone. This is something we did with our ENT resident team and surgical attending, which also let us know we needed to do additional education on how to look for holes in wrappers and how to verify sterilization from the chemical indicator in the tray. For facilities who have residents, you may find they are taught by the resident prior to them, and prior to them, and so on. If you can teach them right the first time, they will start teaching those who follow in their footsteps correctly. Start asking yourself the question, how do my residents get education on an annual or routine basis on all infection prevention items? Once you learn this answer, insert yourself into that process.
In summary, surgeons will become interested in what the infection prevention department is doing when they see there is something that interests them, that IP is invested in them and/or their program or that IP shows them how beneficial and inclusive the programs are for their practice. Gaining trust, building relationships, and putting in the effort up front is key to a successful partnership between infection prevention and our surgeon partners.
Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST, is manager of the Infection Prevention Department at Emory University Hospital Midtown in Atlanta, and is currently co-chair of the AAMI PB70 Committee.
Applying Infection Prevention to Endoscope Evaluations
By Margaret Miller, BS, MT(ASCP)M, CIC, FAPIC; Mairead Smith; and Amanda Sivek, PhD
This article originally appeared in the April 2023 issue of Healthcare Hygiene magazine.
Endoscopy is a valuable diagnostic and therapeutic tool for many clinical specialties, but the technology presents a particular challenge to infection prevention. Long, flexible endoscopes with internal working channels or intricate mechanical elevator mechanisms can harbor contaminants and microorganisms despite best efforts to clean and disinfect them.
Reprocessing can include hundreds of individual steps. The steps are broadly grouped into "point of use" pre-cleaning, leak testing, inspection, manual cleaning (brushing), high-level disinfection or sterilization, rinsing, drying, and finally, storage. Improper handling and storage practices at any point can re-contaminate previously disinfected scopes, heightening the risk of patient infections.
Often, these critical tasks are performed by healthcare workers (HCWs) with limited time, resources, and tools working in cramped spaces with poor ergonomics. Although FDA and experts have repeatedly emphasized the need to adhere closely to a manufacturer’s reprocessing instructions, gaps and variations in practices are common.
Endoscope Reprocessing in the Real World
Through consultations with medical facilities, ECRI has observed some recurring issues, including:
• Confusion regarding expiration dates on test strips – specifically, the shelf life of sealed, unopened test strips as compared to shelf life after packaging has been opened
• Lack of separation between clean and dirty areas in endoscope reprocessing rooms
• Insufficient HCW education and training, including rationale for essential steps
• Use of brushes not recommended by a manufacturer - for example, a toothbrush
• Too few scopes to meet demand
• Failure to plan to replace scopes at the end of their useful life. Scopes require maintenance to remain functional and intact. Damage may inhibit reprocessing and clinical functionality.
• Failure to routinely clean the scope storage cabinets, and
• Storage of endoscopes in procedure rooms – for convenience, or lack of another appropriate storage area.
In addition, ECRI surveyed HCWs who perform duodenoscope pre-cleaning and manual cleaning about their experience. Three-hundred forty-one HCWs responded. Responses showed that:
• Approximately 8 percent of respondents did not have the required equipment to perform their work
• 12 percent of respondents’ facilities do not have a procedure for situations when manual cleaning is delayed; another 12 percent did not know if their facility had a procedure
• Greater than 50 percent of respondents reported experiencing moderate, significant, or excessive discomfort in each of the following areas: lower back, neck, shoulders, and mid-back
To combat these challenges, ECRI recommends that facilities establish reprocessing protocols and policies for HCWs based on professional association guidelines. This will support a safe environment for HCWs and help reduce the risk of infection for patients. All endoscopes are to be cleaned and high-level disinfected or sterilized according to the manufacturer's instructions for use (IFUs).
Further, HCWs should be trained in standard infection prevention and control practices as well as model-specific cleaning and high-level disinfection processes for each endoscope type. Make the IFUs understandable and accessible to your HCWs by posting checklists, graphics, and other memory aids for helping in times of stress (like high production pressure), and for tasks that are not performed often. And remember that IFUs change as suppliers update their recommendations. Consider having the endoscope manufacturer provide annual training or an onsite update when a new reprocessing technique or accessory is introduced. The goal is to have a safe scope to use for every patient.
Possible Technological Solutions to Consider
Publicized outbreaks of multi-drug resistant organisms, linked to ineffective reprocessing of duodenoscopes over the last decade, have helped to drive technological and practice changes across the endoscope landscape. Beginning in 2015, the U.S. Food and Drug Administration (FDA) required manufacturers to conduct post-market surveillance studies to understand duodenoscope cross-contamination. Since then, major duodenoscope manufacturers have introduced new models with single-use endcaps, intended to improve access to the difficult-to-clean elevator mechanism. Other manufacturers have introduced fully single-use models.
In 2022, FDA recommended that healthcare facilities transition to these newer duodenoscope models. ECRI has found that single-use endoscopes can offer advantages for:
• Patients with compromised immune systems
• Patients with a known infection
• Situations when scope reprocessing is unavailable (for example, after hours)
• Facilities that perform endoscope procedures infrequently
However, the cost of single-use endoscopes may be prohibitive for facilities that perform a high volume of procedures, and in some cases, physicians may need advanced features that are not currently supported by single-use models.
In 2021, FDA similarly recommended that facilities consider the use of single-use bronchoscopes “in situations where there is increased risk of spreading infection.” They further recommended that facilities consider routine sterilization of reusable bronchoscopes when feasible to increase the margin of safety, as compared to high-level disinfection.
Facilities looking to transition to sterilizing endoscopes will need to:
• Verify that validated sterilization methods exist for current endoscope inventory, or transition to models that support sterilization
• Ensure that proper sterilization equipment that conforms to endoscope IFUs is available
• Consider any time, space, or cost challenges associated with moving from a high-level disinfection workflow to a sterilization workflow
Conclusion
Endoscope reprocessing is mission-critical, labor-intensive, meticulous, and often time-sensitive work. Staff may not have access to all the resources they require. Despite when reprocessing guidelines are carefully followed, cross-contamination continues to occur. Reprocessing recommendations and technology continue to evolve. Infection preventionists, clinicians, and healthcare facilities need to make informed decisions about the latest developments to keep progressing towards our joint goal of preventing pathogen transmission from patient-to-patient.
Margaret Miller, BS, MT(ASCP)M, CIC, FAPIC, is an infection preventionist at ECRI.
Mairead Smith is principal project engineer I at ECRI.
Amanda Sivek, PhD, is principal project engineer I at ECRI.
References:
1. Healthcare worker views: duodenoscope reprocessing workflow and ergonomics. Device Evaluation 2021 Jun 9.
2. Sivek AD, Davis J, Tremoulet P, Smith M, Lavanchy C, Sparnon E, Kommala D. Healthcare worker feedback on duodenoscope reprocessing workflow and ergonomics. Am J Infect Control. 2022 Jan 30: S0196-6553(22)00055-4. Doi: 10.1016/j.ajic.2022.01.012. Epub ahead of print. PMID: 35108583.
3. Poor Duodenoscope Reprocessing Ergonomics and Workflows Put Healthcare Workers and Patients at Risk: Hazard #8—2022 Top 10 Health Technology Hazards. Device Evaluation 2022 Jan 12. For members: Top 10 Health Technology Hazards for 2022: Solutions Kit (ecri.org)
Executive brief available at: Top 10 Health Technology Hazards for 2022 Executive Brief (ecri.org)
The Role of Evidence-Based Guidelines and Consensus Documents: What is Required?
By Sylvia Garcia-Houchins, MBA, RN, CIC
This article originally appeared in the March 2023 issue of Healthcare Hygiene magazine.
Healthcare organizations (HCOs) preparing for survey understand they will undoubtedly be asked to reveal which evidence-based guidelines (EBGs) their facility uses. This is a staple of survey, whether conducted by The Joint Commission, the Centers for Medicare and Medicaid Services (CMS) or a state since all require the use of EBGs.
The most accurate way to prepare for and respond to this EBG inquiry during survey is to:
• Identify which EBGs and consensus documents are required vs. those that are optional
• Document and disclose basic processes used for determining required elements
How many EBGs are required?
The Joint Commission does not require a minimum number of EBGs. HCOs that receive CMS deemed status through The Joint Commission can locate required EBGs within program-specific CMS State Operations Manuals. For example, the Ambulatory Surgery Center (ASC) State Operations Manual states, “The infection control and prevention program must include documentation that the ASC has considered, selected and implemented nationally recognized infection control guidelines.”
During an ASC deemed status survey, Joint Commission surveyors complete a form to identify selected guidelines and must confirm a selection or score non-compliance “even if the ASC’s infection control practices comply with generally accepted standards of practice/national guidelines.”
The survey form lists potential guidelines ASCs may select, including:
• The Centers for Disease Control and Prevention’s (CDC) isolation, hand hygiene, environmental, or disinfection and sterilization guidelines
• The Association of periOperative Registered Nurses (AORN) Perioperative Standards and Recommended Practices
• Specialty surgical societies’ guidelines
Each surveyor must indicate at least one infection control-related EBG by name during survey.
During the guideline selection process, while it is critical infection preventionists understand there is no specified number of EBGs to consider, they must demonstrate their facility has reviewed and selected EBGs to implement. Additionally, they must maintain an active and organization-wide infection control program consistent with nationally recognized infection prevention standards.
Which EBGs are required by The Joint Commission and/or CMS?
During every Joint Commission survey, compliance is evaluated with:
• CDC and/or World Health Organization (WHO) 1A, 1B and 1C hand hygiene guidelines (NPSG.07.01.01 EP1)
• CDC isolation guidelines (IC.02.01.01, EP 2 and 3 on standard and transmission-based precautions)
Joint Commission standards and CMS requirements are not prescriptive regarding other EBGs or consensus documents that HCOs must consider or implement. CMS recently clarified this within its interpretive guidance chapters:
• Hospitals have ample recognized evidence-based approaches to select from in order to adhere to nationally recognized guidelines without impeding their ability to otherwise make progress in infection prevention and control.
• ASCs must select one or more sets of guidelines that enable them to address the key functions of an effective infection control program.
Joint Commission standards contain general wording that specifies an HCO should “measure and monitor its infection prevention processes, outcomes and compliance using EBGs or best practices, and consider EBGs when implementing evidence-based practices.”
Are there any other requirements for EBGs?
Joint Commission-accredited HCOs that have considered, selected and implemented nationally recognized infection control guidelines should be considered compliant as long as there are not manufacturer instructions for use (IFUs) or state regulations that otherwise specify compliance with a particular EBG or consensus document.
Joint Commission standards require HCOs comply with state law and regulation and follow manufacturer IFUs as part of a hierarchical method to address infection control-related requirements.
Several states have adopted specific EBGs or consensus documents by incorporating them into healthcare code requirements. It is key HCOs access their specific state requirements as they vary from state to state. For example:
• Illinois: Requires long-term care (LTC), ASCs and hospital settings to adhere to specific CDC guidelines ,
• New Jersey: Provides an exception to the adoption of specific CDC guidelines by LTC if there is sound infection control rationale based upon scientific research or epidemiologic data. Additionally, specific Advancement of Medical Instrumentation (AAMI) and Society of Gastroenterology Nurses and Associates (SGNA) standards are required for central services.
• Texas: Requires ASCs to develop policies and procedures for sterile supplies based on standards, guidelines and recommendations by AORN, Association for Professionals in Infection Control and Epidemiology (APIC), CDC, and if applicable, SGNA.
Infection preventionists may access statutes, codes and regulations by state but should be cautious to ensure they have considered all applicable requirements based on their unique healthcare setting and confirm requirements with the source and/or legal counsel.
Additionally, manufacturer IFUs may direct users to reference EBGs and consensus documents for further information. HCOs must ensure EBGs are used to clarify additional requirements and not to supersede the IFU.
If an EBG is not required, how can infection preventionists assess whether it should be incorporated into their infection control program?
It is essential for infection preventionists to pay careful attention to sources and processes used to develop recommended infection control practices. They should know the difference between an EBG, guidance, consensus documents, position statements and policies, as well as the processes used to develop these documents so they can make educated choices.
• EBGs: Developed to answer questions via a literature search protocol which identifies relevant articles. The evidence in those articles is then abstracted and summarized before a group assesses it and formulates recommendations based on consensus. Before approval and publication, factors assessed include impact, feasibility, and risk and benefits. EBGs should provide references for the user to evaluate relevance and context specifically for their HCO.
• Guidance: Meant to provide instruction on how to address a situation and may include relevant literature. However, guidance documents may not consider unique aspects of each HCO and must be carefully evaluated to determine risk and benefits to HCOs that follow the guidance.
• Consensus documents: Created by a group and represent those individuals’ collective opinions, which may or may not be supported by scientific literature. If a consensus group follows the American National Standards Institute (ANSI) and agrees to its oversight, procedures, approval process and more, their resulting consensus document becomes an American National Standard. ISO standards are an example of consensus documents developed through the consensus of experts from many countries and approved and published by a globally recognized body. Infection preventionists may need to review literature to ensure it supports recommendations made in consensus documents.
• Position statements: Provide viewpoints of a professional organization on a particular topic, as well as background and rationale to support that viewpoint. Infection preventionists may need to do their own literature review to determine if the viewpoint is sound or could be negated by additional information.
• Policies: Represent both how an HCO interprets relevant requirements and how it implements them.
Misinterpreting EBGs, guidance, consensus documents and position statements as requirements and adopting policies of other organizations with differing requirements, especially across state lines, has led to significant misunderstandings regarding Joint Commission and CMS requirements. Such misinterpretations can result in regulatory, financial and resource implications for HCOs Infection Preventionists must clearly articulate to surveyors which EBGs are required within their state or manufacture instructions and which EBGs or other documents have been incorporated by choice.
Which part of an EBG is required by The Joint Commission?
Unless required by Joint Commission standards, regulation or manufacturer instructions, HCOs may choose which segments of EBGs and consensus documents to incorporate into their practices. To help identify the appropriate recommendations to use, HCOs should consider level of recommendation or word choice, for instance:
• CDC (use of rankings): 1A and 1B recommended, 1C required, and II suggested
• AORN (use of a word within context of the guidelines): Should indicates action is recommended; must describes requirements mandated by regulation; may indicates action is permissible within the limits of the guideline; and can indicates possibility and capability.
• Association for the Advancement of Medical Instrumentation (AAMI) (use of verbal terms within documents to distinguish its requirements): Shall and shall not express requirements; should and should not express recommendations; may and may not express permission; can and cannot as statements of possibility or capability; might and might not express possibility; and must signifies external constraints or obligations defined outside the document.
It is important to review rankings and terms provided by authors seek clarity of a perceived requirement from the authoring organization.
Infection preventionists need to clearly understand when, which and what part of EBGs are required before selecting a particular EBG or another document to incorporate into their infection control policies, protocols or processes. They should add recommendations above the basic requirements from EBGs to organizational requirements only if they are based on compelling evidence that the improve safety or quality, are feasible, and cost effective. Requirements based of optional EBGs should never be added if they conflict with routine organizational practices.
An ideal EBG process is one that includes multidisciplinary input and evaluation of EBGs and other documents before they are incorporated into policies. Since leadership is responsible for the development and implementation of organizational policies, there should be a process to ensure leadership agrees with the incorporation of optional EBGs. This helps an HCO avoid being scored for non-compliance during survey and allows it to explain which and what part of EBGs have been incorporated into its infection control program.
Sylvia Garcia-Houchins, MBA, RN, CIC, is director of infection prevention and control for The Joint Commission.
References:
1. The Joint Commission. Clarifying Infection Control Policy Requirements. Perspectives. April 2019.
2. Illinois Administrative Code. Title 77 § 300.696 available at Section 300.696 - Infection Prevention and Control, Ill. Admin. Code tit. 77 § 300.696 | Casetext Search + Citator Accessed February 25, 2023
3. Illinois Administrative Code. Title 77 § 250.105 available at Section 250.105 - Incorporated and Referenced Materials, Ill. Admin. Code tit. 77 § 250.105 | Casetext Search + Citator Accessed February 25, 2023
4. New Jersey Administrative Code. Section § 8:43G-14.1. Available at Section 8:43G-14.1 - Infection control program structural organization, N.J. Admin. Code § 8:43G-14.1 | Casetext Search + Citator Accessed February 25, 2023.
5. Texas Administrative Code. Section 135.11 Available at https://casetext.com/regulation/texas-administrative-code/title-25-health-services/part-1-department-of-state-health-services/chapter-135-ambulatory-surgical-centers/subchapter-a-operating-requirements-for-ambulatory-surgical-centers/section-13511-anesthesia-and-surgical-services Accessed February 25, 2023.
Revised Liquid Barrier Standard Reflects Real-Life Experiences, Needs of End Users
By Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST
This article originally appeared in the February 2023 issue of Healthcare Hygiene magazine.
The Association for the Advancement of Medical Instrumentation (AAMI) has released an updated version of the American National Standard of personal protective equipment (PPE) in the healthcare environment. This standard, ANSI/AAMI PB70:2022, Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities, has been revised for the first time since the last release in 2012. Why update now? What has changed? Quite frankly, a lot. As healthcare providers, representatives from professional organizations, and manufacturers, the committee has incorporated a vast amount of knowledge to ensure the standard reflects real-life experiences and needs of end users.
A main driver for the revisions centered on the limited variety of gowns that were listed in the document. The committee members wanted to expand the choices to allow for more specific personal protective equipment (PPE) to be selected for individual tasks and jobs. By expanding the definition of the choices of gowns, as well as updated the labeling requirements to ensure clear labeling of the type of gown you are choosing, the end user will be provided a safer environment for their work.
A new category of surgical gown was introduced, labeled “Surgical Gown-E.” This gown specifically provides extended protection in the critical zones due to the nature of certain procedures and actions that may require a higher level of protection from potentially infectious materials.
Additional explanation of procedure gowns, such as definition protection for open-back gowns, non-protection back gowns, and decontamination gowns were also added to assist the end-user in making an informed choice on what gown to wear during specific tasks.
The decontamination gown for sterile processing professionals is a new category that is of particular interest to those who have ever worked in sterile processing and/or at a decontamination sink. Have you ever taken off your PPE after working several hours in the decontamination area and your scrubs underneath are wet from strike-through? Were you wearing an appropriate gown? One of the bigger questions is—do you know what an appropriate gown for working in the decontamination area is? Now, with the new updates to PB70, this is very clear. Decontamination gowns must be a minimum of an AAMI Level 3; how can end users use this to move forward with the new standards immediately? Until manufacturers begin marketing new gowns with this label, end-users should check their current gowns to be sure they have gowns with this minimum level of protection for their team members.
Future plans and work items for the PB committee include developing a standard for emergency preparedness and PPE practices by gathering data and information from the lessons learned during the COVID-19 pandemic. The group will also focus on updating the current TIR11 (Technical Information Report) document to assist end users with implementing the PB70 document into the daily practices.
Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST, is manager of the Infection Prevention Department at Emory University Hospital Midtown in Atlanta, and is currently co-chair of the AAMI PB70 Committee.
The author acknowledges Cheron Rojo, BS, FCS, CHL, CIS, CER, CFER, CRCST, co-chair of the AAMI PB70 Committee, for editing assistance with this article.
Symposium on Infection Prevention & Control Tackles the Tough Issues Facing the Profession
This article originally appeared in the November 2022 issue of Healthcare Hygiene magazine.
The virtual Symposium on Infection Prevention & Control, held Nov. 1-3, 2022, developed by Healthcare Hygiene magazine and Keystone Media Inc., explored some of the most pressing issues facing the profession and provided experts’ perspectives on tackling perennial issues with innovative strategies. A limited number of seats for this symposium is still available; RSVP at: https://inevent.com/en/KeystoneMediaInc/SymposiumonInfectionPreventionandControl/form.php
***
In her presentation addressing controversies and conundrums in infection prevention and control, Connie Steed, MSN, RN, CIC, FAPIC, reviewed and reflected on healthcare infection prevention and control during the past 50 years while describing the current state of the profession.
As Steed noted, “I think we continue to struggle with rebuilding the resilience of the patient safety culture that we had prior to the COVID-19 pandemic. We have lost our sustainable healthcare-associated infection prevention status that we had pre-COVID at the frontline of care. And so today, we're still working on getting back those successful, sustainable prevention methods.”
Steed acknowledged the numerous challenges facing today’s IP&C programs, including staffing challenges, an aging workforce and the threat of burnout, as well as resourcing challenges, supply chain issues and the struggle to follow manufacturers’ instructions for use (IFUs). She pointed to a shift in how people think about infection transmission post-pandemic, as well as the escalating expectations and demands of the profession.
In her presentation, Steed outlined the challenges and conundrums associated with each of several big issues, including the infection prevention workforce; IP&C program infrastructure variation, including organizational IP&C decision making and priorities; sustaining best practice, including healthcare provider compliance and accountability with IP&C standards of practice; HAI prevention and control innovation, including evidence-based practice and early adaption with grey literature; as well as addressing emerging pathogens that are impacting the profession. Steed also discussed strategies to address these issues to make way for progress in a post-COVID world.
***
Robert Garcia, BS, MT(ASCP), CIC, FAPIC, reviewed the 14 imperatives that will impact infection prevention and control post-COVID and into the future that were recently published in AJIC as a paper titled “Recommendations for Change in Infection Prevention Programs & Practice.”
Garcia touched upon several key areas from this paper, environmental contamination, diagnostic stewardship, and bloodstream infections, and shared his thoughts on the current guidance for addressing critical issues currently affecting IP programs with an emphasis on the implementation of innovative, cost-effective, and evidence-based interventions, engaging healthcare leaders and experts in clinical care in proven prevention measures, holding staff accountable, and adopting high reliability principles.
“The advances must be accomplished with the understanding of the importance of a structure for infection prevention nationally that spans across the continuum of care from acute to skilled nursing to ambulatory to post-acute settings and which is resilient to mammoth events such as pandemics,” he said. “Regardless of which strategies are considered, infection prevention successes will depend largely on strong leadership support.” He pointed to three practices as important facilitators in the prevention of HAIs; the first involves engagement of executive staff. “Establishment of infection prevention goals by executive leadership emphasizes an organizational priority among managers and frontline staff and enables open communication with persons who are empowered to make change.” The second addresses information sharing: “Establishment of an organization-wide system to relay, display, and discuss relevant infection data with frontline staff is an important activity.” The third involves management coaching “The coaching activities identified as most needed involve providing staff with feedback on how to perform clinical care processes correctly and re-educating staff on best practices for infection prevention.”
Garcia added, “The future success of infection prevention programs lies in identifying and implementing cutting-edge program modifications and best practices while supported by targeted executive actions.”
***
Barbara DeBaun, MSN, RN, CIC, addressed taking infection prevention practice to the next level, providing an inspirational examination of the IP&C profession.
“So, does anybody ever say to you, ‘What exactly do you do?’ or ‘What is an infection preventionist?’ DeBaun asked during her presentation. “I certainly get asked that question a lot, and three years ago when the pandemic reared its ugly head, I was certainly asked a lot about what I do. When you think about it, it's important to be prepared as to how to answer that question. I would encourage you, if you haven't done this recently, to take a good long hard look at your current job description. Does it accurately reflect what you do? Does it elevate your position? Do you have words like ‘conduct surveillance’ or ‘performs hand hygiene audits’? Or are you using elevated terms that are more about ‘driving improvement on interpreting data,’ for example? It’s important for us as IPs to ensure our job descriptions tell the story of what we really do.”
DeBaun continued, “I would encourage you to think about how to customize your ‘elevator speech.’ This is a term we use a lot and what it means is if you get on the elevator and someone on that elevator asked you something that you had to explain in the short time it takes the elevator to reach its destination. Would you be prepared to respond in a meaningful, memorable way? You've got a name tag that says ‘infection prevention’ but people might be curious about what is it that you do. I would encourage you to think differently based on who's asking the question; is it a family member who's there to visit a loved one? Is it your system’s chief medical officer or maybe the chief financial officer? Perhaps it could be another staff member? Your response must be different based on who's asking the question.”
DeBaun added, “When asked ‘what do you do as an infection preventionist?’ one of my colleagues without even thinking about it blurted this out and I've never forgotten it. She said, ‘Well, we try to get people to do things they don't want to do and then get them to think it was their idea in the first place.’ She said it to be funny but when you think about it, that's exactly what we do. As infection preventionists, we are influencers and engagers who try to get people to do things that they probably didn’t wanted to do in the first place, but get them so excited about it that they're starting to think they came up with the idea in the first place. So, our goal as IPs is to be an effective influencer, to get others to care about the role of infection prevention, so that we can elevate our practice.”
***
Janet Haas, CEO and principal consulting epidemiologist at Innovative Infection Prevention, addressed the roles of infection preventionist and healthcare epidemiologists within the context of challenges that they face daily. She reviewed the CMS requirements in its conditions of participation requiring a person or persons designated as infection control officer or officers to develop and implement policies, governing the control of infections.
“When you think about our profession and what background we need, what entry-level training is required, and what is required for ongoing education and training, anybody who's been in this field knows that this is quite a mishmash traditionally,” Haas said. “It's been mostly nurses working as IPs, but that's changed a lot in recent years, with people coming from many different backgrounds, such as MPHs or even microbiologists. There's not a specific requirement for this, unless your state has requirements for you as a licensed professional in some cases, but there's not one single standard certification. To me, the CMS description closely matches what most infection preventionist are doing, it matches the effect and impact they are having, describes our professional practice and standards for professional healthcare epidemiology programs.”
Haas continued, “We have some work to do there, and IPs need a Department of Labor classification. By contrast, if you ask, what's an epidemiologist, you can see that there is a Department of Labor definition that says an epidemiologist is a public health worker who investigate patterns and causes of disease and injury. Sounds a lot like what we do. So, I think that it is interesting because sometimes there is a bit of a push and pull between the epidemiologist role and the infection preventionist role.” Haas proceeded to explore the history of this duality, beginning with the results of the SENIC study from the 1970s.
Haas acknowledged that, “In an ideal world, we would define the roles, responsibilities and requirements for infection preventionists and healthcare epidemiologists that would transcend politics and focus on leadership and skill sets.”
Haas recommended that IPs set and review their professional goals, revisiting them annually and whenever a big change occurs, as well as advised IPs to be honest with themselves about their desire and ability to continue or change course. She also recommended IPs assess their current work situation to ensure that it improves their chances of success within the organizational culture and organizational goals, as well as consider their professional growth trajectory.
***
In her presentation addressing the interactions of infection preventionists and non-clinicians, Connie Cutler, RN, BSN, MS, CIC, FSHEA, FAPIC, reviewed how infection prevention teams interact with non-clinicians in typical scenarios, such as during product evaluation and purchasing situations. She noted that the infection preventionist is often highly sought by company sales representatives, and that it can be a good opportunity to learn about products and technologies in the marketplace, but that parameters for interaction must be established. “I've had very good working relationships with them over the years, creating collegial relationships, which is important,” Cutler said. “They often can help you trial their technologies for free, which can be very beneficial to the institution’s bottom line. But the days of having the sales rep just knock on the door and say, ‘Do you have 5 minutes to talk,’ are gone. That used to happen frequently in my career, and if I could, I used to talk to them, but many times I would say, ‘I would appreciate you making an appointment because this is not a good time.’ They had been in the facility to see somebody else and had thought while they're there, they would stop and talk to the IP. But nowadays, that's inappropriate. They need to make an appointment, and I need to find out if their product is even on our purchasing agreement before I visit with them, unless they have something so unique that it's a one of a kind.”
Cutler added, “Purchasing is also a good colleague to develop, if you haven't already. Purchasing personnel have the goal of saving the hospital money, but over my 40 years, I've worked with them enough that they understand the big picture -- if we prevent infections, that saves the hospital money. It used to be that everybody had their own little bucket of money and purchasing would say, ‘Well, if you want to buy product X that costs more than product Y, then you must prove to me why this would make sense.’ And so, over the years I've worked with them, I share data internally about how bringing in that product has saved other hospitals money and that whole messaging is made much easier. Now, they understand that it's not just their bucket of money, it's the hospital's bucket of money. And so, over the years it's been a lot easier to bring in new products which will prevent infection.”
Cutler also discussed how the concerns of non-clinicians about infection-related matters can be best addressed, as well as reviewed communication guidelines for infection preventionists and external colleagues.
***
Cindy Fronning, RN, GERO BC, IP BC, AS BC, RAC CT, CDONA, FACDONA, EFLA, CALN, director of education for NADONA, addressed the current state of long-term care infection prevention in her presentation, noting, “When I think about what could be possible in the future, there is so much to look forward to. The role of the infection preventionist is one that's really growing in long-term care, and with guidance that has changed for the infection preventionist program, there is more opportunity than ever before to prevent infections in long-term care settings.”
Fronning reviewed the past and pre-COVID aspects of long-term care, emphasizing that prior to 2017, there hadn’t been as strong a focus on the role of the IP as there is now, due to new regulations effective Oct. 24, 2022 requiring at least one person being designated as an IP and tasked with infection prevention accountability in LTCFs. Fronning reviewed these new CMS requirements in detail, emphasizing that an infection prevention mindset will be required for future success, especially as facilities emerge from the pandemic having learned painful lessons that can be applied to practice today.
She encouraged long-term care facilities to examine their current infection rates, study the CMS regulations to stay in compliance, and to make a concerted effort to implement the requirements “to
ensure a safe and healthy future for all.”
***
Carol Calabrese, RN, BS, T-CSCT, CHESP, CIC, emphasized that across the healthcare delivery continuum beyond acute care, infection prevention and control encompassed a number of “constants” that applied to all care settings, including ambulatory care, long-term care, dialysis centers, home healthcare, and others. These constants include knowledge of the chain of infection, hand hygiene, environmental cleaning and disinfection, safe injection practices, and many others. She examined the critical IP&C-related challenges in the non-acute setting(s) and shared recommendations for these settings. She addressed the lack of support for infection prevention and a dearth of administrative support for IP&C programs, as well as staffing issues, management of IP&C in ancillary settings, varying degrees of oversight, as well as the need for consistent antimicrobial stewardship in all clinical arenas.
Calabrese advocated for national and/or state standards for a required number of infection preventionist FTEs, as well as surveys beyond long-term care, dialysis, and home health, as well as
consistency in training and certification, and the awareness that all physician offices, urgent care chains, pain clinics and other sites need to have designated IP&C programs and an active IP on site.
***
In their presentation exploring the intersection between infection prevention, quality outcomes and healthcare value analysis, Judy Allison, MBA, and Jim Davis, MSN, RN, CCRNK, CIC, HEM, FAPIC, shared insights relating to the value analysis journey. They emphasized the importance of an organizational culture that embraces the Triple Aim in healthcare, encompassing quality, cost and value, and how these imperatives can be applied to the clinical and fiscal decision-making process.
They explained the importance of the collaboration of a multi-disciplinary team with decision-making authority, noting that the service line focus of the team allows for focus, SME input to this decision-making and implementation “buy in” from all stakeholders, including service line leaders, physicians and clinical users, finance, quality and risk, supply chain and infection control.
***
Maggie Miller, MT(ASCP) M, CIC, FAPIC, Erin Sparnon and Mairead Smith discussed their perspectives on applying real-world infection prevention to ECRI’s endoscope evaluations, where they discussed how to best navigate the shifting product and regulatory landscape while sharing clinical and engineering observations and concerns related to endoscope assessment. They also identified key use cases where single-use endoscopes and endoscope components may offer meaningful benefits to patients and healthcare facilities. Additionally, they shared eye-opening results from a recent duodenoscope reprocessing survey that addressed key workflow- and ergonomics-driven imperatives. They shared a response from an acute-care facility respondent who observed, “Right now, PPE depends on what is available on that day. It's a crapshoot if we will have what we need and if it is the right size. Same goes for other cleaning supplies. Supply is unreliable. We often have to 'make do.' We are also the last thought. Sometimes it seems as if we are forgotten about in the basement as our water can get shut off without warning, along with electricity.”
***
The November Symposium on Infection Prevention & Control also featured encore presentations by Sylvia Garcia-Houchins and Cori Ofstead, who presented at the October Symposium on Sterile Processing & Infection Prevention, also developed by Keystone Media Inc. and the Healthcare Sterile Processing Association (HSPA).
Sylvia Garcia-Houchins, MBA, RN, CIC, explained The Joint Commission survey process, focusing on the sterile processing departments of acute-care facilities. She provided examples of situations that could lead to survey findings in SPDs and adverse accreditation decisions, as well as described the approach for ensuring compliance with Joint Commission infection control standards. She also reviewed the differences between the roles of evidence-based guidelines and consensus documents in the accreditation process. She reminded attendees that guidelines that have been scientifically developed based on recent literature review and are consensus driven, while a voluntary consensus standard is a type of standard developed or adopted by voluntary consensus standards bodies using a development process characterized by openness, balance, due process, consensus, and the right to appeals.
Cori Ofstead described the evidence supporting standards and guidelines for training and certification for sterile processing professionals while explaining the new recommendations for visual inspection of flexible endoscopes. She also shared new evidence on splashes and droplet dispersal in sterile processing areas and suggested practical ways to mitigate splash exposure and protect sterile processing personnel from contamination. The study, published in the American Journal of Infection Control, showed that manual cleaning of reusable medical instruments generates substantial splash more than seven feet from the source. The findings reinforce the importance of engineering controls and appropriate use of personal protective equipment (PPE) to reduce the risk of contamination for processing personnel and their facilities.
“This study confirms that technicians working in sterile processing departments are at risk for exposure to water droplets that may contain blood, tissue and other patient fluids,” said Ofstead, president and CEO of Ofstead & Associates, and the paper’s lead author. “Even in a sterile processing department optimally designed to reduce potential exposures, we were surprised by the abundance of droplets generated during the performance of instrument processing steps that were completed in accordance with manufacturers’ instructions for use.” The study expands upon a pilot project completed by Ofstead and colleagues in 2021 - the first real-world evaluation of PPE effectiveness for sterile processing personnel – which concluded that personnel who process reusable medical instruments and equipment may be exposed to tissue, blood, and other patient fluids even when wearing recommended PPE. Previous research has demonstrated a link between contamination disseminated from sinks and healthcare facility outbreaks.
Tracer Methodology: An Important Infection Control Tool for Identifying Risk
By Sylvia Garcia-Houchins
This article originally appeared in the October 2022 issue of Healthcare Hygiene magazine.
Each infection prevention and control program within healthcare organizations across the nation is unique. All infection prevention programs should share similar foundation and structure, but each individual program is shaped by its healthcare organization’s unique culture, physical location(s), and staff. Additionally, each program contains various combinations of care treatment, services, supplies, products, and equipment.
It is the uniqueness and ever-evolving nature of each healthcare organization that validates the importance of periodically using The Joint Commission’s tracer methodology as a diagnostic tool. Tracer methodology is the process that Joint Commission surveyors use to analyze a healthcare organization’s systems or processes for delivering safe, high-quality care. It is used to identify real and potential patient safety risks.
By periodically using tracers, infection preventionists can not only monitor for compliance with set requirements (checklist approach) but can also use a broader review to identify and assess variations in processes and practices. Some variations are completely acceptable, while others are potentially dangerous and could put healthcare organizations, staff, patients, and visitors at risk.
While using tracer methodology Joint Commission surveyors analyze processes by following an individual patient through a healthcare organization’s care processes in the sequence experienced by that individual. The tracer methodology process may require visiting just one or multiple care settings to review the care rendered and the systems to support that patient’s care. As often witnessed during survey, tracer methodology helps identify varying level of risk – including some that pose an immediate threat to a patient’s health or safety and require immediate action to mitigate the risk.
An infection preventionist can use tracers to identify risk that may have resulted in a patient developing a healthcare-associated infection, to proactively identify risk that could result in an infection, or to review a newly implemented risk-reducing healthcare system.
For example, an infection preventionist could trace a patient with a central venous catheter (central line, trace the care of a patient who developed a central line-related bloodstream infection or trace a system that has been implemented to decrease risk of central line infection.
By tracing the patient’s locations beginning with where the initial decision was made to insert a central line, to the location of insertion, to each location where it is used or accessed, infection preventionists can identify risks that could be acted upon to decrease the risk of central line related bloodstream infection for future patients.
Important aspects of a good tracer process include:
- Invite variety. Do not follow the same tracer path every time
- Always keep an open mind when collecting tracer data
- Ask open ended questions that lead to a follow-up question or create a “show me?” moment
- Collect information that can be further explored– either immediately or when time allows
- Unless a patient is at immediate risk, do not immediately begin remedying – the purpose is to collect tracer data
- Do not place blame during tracer processes
Example scenario of tracing a patient
To better illustrate the tracer process, consider the basic example of an individual patient tracer utilized to trace a patient with a central line. Tracers can begin anywhere, but for this example scenario we first visit the healthcare facility’s oncology floor to identify a patient with a central line.
Then, we review her medical records. Medical records indicate the patient needed a central line for administration of chemotherapy, and subsequently, she had it inserted in the interventional radiology department. (Patient education is documented by the clinic before the insertion, and by radiology before and after the procedure.) Further tracing shows that the patient’s central line has been accessed at the hospital’s infusion center emergency department, inpatient oncology unit, and general medicine unit as well as the hospital’s off-site infusion center.
Next step, the patient is interviewed. She explains that she was overwhelmed by her diagnosis and depends on her husband to remember what she is supposed to do. She remembers being told that she would need a central line placed for chemotherapy and subsequently had it inserted the next week in Radiology. Placement of a central line was not presented as an option, but as a mandatory step, if a patient is to receive chemotherapy. Staff explained the procedure and directed her not to shower until the insertion site was healed. She was provided written instructions at her first chemotherapy visit the following week with direction to look out for redness, swelling, pus, and fever. She was also given direction to call the clinic or an after-hours phone number to report any problems.
Since the inception of the patient’s treatment, nurses in outpatient chemotherapy used a kit to access the central line but since she was admitted, no kit was available. Similar supplies were used when accessing the central line to administer medications, however the technique varied a bit. The emergency room (ER) nurse used alcohol before carefully accessing the patient’s central line. She drew blood for tests yet seemed very nervous. The patient noted that nurses who accessed her central line usually wear a mask, but few requested the patient also wear a mask.
The patient understands that infection of her central line is a significant risk, and she needs to protect it, however she is unsure of what the nurse who gave her the written instructions meant by directing her to protect her line. When asked to role play what she would do if a healthcare professional accessed her central line incorrectly, the patient says she assumes that staff know what they are doing, Further, she says she would never question the care provided by a health professional and doesn’t want to be seen as a difficult patient.
When retracing all locations, the patient has visited, we discover that there are two types of central line access kits in use – one kit includes chlorhexidine containing skin preparation and the other includes plain alcohol. Nurses are allowed to choose according to preference, but most use the chlorhexidine product because it is known to better disinfect. We also find that the healthcare facility’s general medicine unit and emergency room do not stock central line access kits. These areas, instead, gather patient supplies as necessary.
Interviews with staff outside of the infusion areas and oncology unit identify varying levels of experience working with central lines and some nurses say they have yet to complete a competency assessment but have been taught by a colleague, key steps of central line access. One of the nurses in the ER says she asks for oncology staff to access a patient’s central line if it looks like that patient will be admitted. Otherwise, the nurse insists that physicians order a peripheral blood draw and if necessary, a peripheral IV. Undoubtably, the tracer information collected shows that considerable variation exists regarding central line dressing protocol, depending on the location.
Observations from this tracer example scenario highlight variations in practice, supplies, education, and competency of staff caring for patients with central lines. In this example case, provided patient education failed to achieve its desired objective, since the patient is uncertain of the healthcare organization’s approved process for accessing her central line and does not feel empowered to speak up if staff deviates from the process.
Information from this tracer could be used in multiple ways, including:
- Prioritizing tracer information based on risk and using it to develop an infection prevention and control plan for patients with central lines
- Utilizing tracer information as a starting point for more in-depth study and resolution of an issue Exploring how to improve patient education and empower patients to speak up
- Providing data results to a performance improvement team to plan and implement a rapid- cycle plan to improve supply chain issues
- Analyzing root causes to determine why implemented processes have not been sustained
Opportunities to use tracer information are only limited by the staff member who collects it. Often infection preventionists want to continue to collect more extensive data to prove that an issue is real, however they fail to act, because they want their “data” to be perfect. Occasionally, that approach may be appropriate, but at other times, action immediately needs to be taken.
Using tracer methodology may move some infection preventionists out of their routine “surveillance” comfort zone. Others recognize it provides opportunity for positive interaction with patients and staff and helps identify risk that might not have been detected though routine surveillance, compliance rounding, or use of checklists. Tracer methodology is one more tool infection preventionists can use to identify risk for infection. It is worth giving tracer methodology a try.
Access more in information about The Joint Commission’s tracer methodology here.
Sylvia Garcia-Houchins is director of infection control and prevention, Office of Quality and Patient Safety, The Joint Commission.
My Patient Has a Diagnosis of Monkeypox, What Do I Need to Know?
By Margaret M. Miller, BS, MT(ASCP) M CIC FAPIC, and Susan Singh, MPH, CIC
This article originally appeared in the September 2022 issue of Healthcare Hygiene magazine.
What is Monkeypox?
Monkeypox is caused by the Monkeypox virus which belongs to the Orthopoxvirus genus in the family Poxviridae. Monkeypox is usually a self-limited disease with the symptoms lasting two to four weeks. There are two distinct genetic clades (strains) of the Monkeypox virus: the Central African (Congo Basin) clade and the West African clade. The current 2022 outbreak is of the West African clade and is typically associated with less severe illness. More than 99 percent of people who get this form of the disease are likely to survive. However, people with weakened immune systems, children under eight years of age, people with a history of eczema, and women who are pregnant or breastfeeding may be more likely to get seriously ill or die.
What do I need to know about Monkeypox?
Monkeypox can spread from person-to-person through:
• Direct contact with the infectious rash, scabs, or body fluids
• Respiratory secretions during prolonged, face-to-face contact, or during intimate physical contact, such as kissing, cuddling, or sex
• Touching items (such as clothing or linens) that previously touched the infectious rash or body fluids
• Pregnant women can spread the virus to their fetus through the placenta
If you have cared for a Monkeypox patient, be alert to the development of symptoms (fever, chills, new rash, or lymphadenopathy) for 21 days after the last date of care, and notify infection control, occupational health, and the health department to be guided about a medical evaluation.
• Healthcare workers who have unprotected exposures (i.e., not wearing PPE) to patients with Monkeypox do not need to be excluded from work duty, but should undergo active surveillance for symptoms, which includes measurement of temperature at least twice daily for 21 days following the exposure. Prior to reporting for work each day, the healthcare worker should be interviewed regarding evidence of fever or rash.
• Healthcare workers who have cared for or otherwise been in direct or indirect contact with Monkeypox patients while adhering to recommended infection control precautions may undergo self-monitoring or active monitoring as determined by the health department.
What do I need to do differently for a patient with Monkeypox?
Notify the infection prevention and control department that a patient with signs and symptoms of Monkeypox has been admitted.
Have a policy in place that is customized to your facility’s needs and outlines the management of patients with suspected or confirmed Monkeypox.
Transmission-based precautions and patient placement
• Patients with suspected or confirmed Monkeypox infection should be placed in a single-person room; special air handling is not required. The door should be kept closed (if safe to do so). The patient should have a dedicated bathroom.
• Transport and movement of the patient outside of the room should be limited to medically essential purposes. If the patient is transported outside of their room, they should use well-fitting source control (e.g., medical mask) and have any exposed skin lesions covered with a sheet or gown.
• Intubation and extubation, and any procedures likely to spread oral secretions, should be performed in an airborne infection isolation room (AIIR)
• Use of portable fans, dry dusting, sweeping, or vacuuming should be avoided to prevent re-suspending the dried material from lesions
• Ensure signage is posted on patient’s door with the appropriate transmission-based precautions
Personal protective equipment (PPE)
PPE used by healthcare personnel who enter the patient’s room should include:
• Gown
• Gloves
• Eye protection (i.e., goggles or a face shield that covers the front and sides of the face)
• NIOSH-approved particulate respirator equipped with N95 filters or higher
Waste management
Waste management (i.e., handling, storage, treatment, and disposal of soiled PPE, patient dressings, etc.) should be performed in accordance with U.S. Department of Transportation (DOT) Hazardous Materials Regulations (HMR; 49 CFR parts 171-180.)
Required waste management practices and classification currently differ depending on the Monkeypox virus clade.
• West African Clade (current 2022 outbreak): Waste contaminated with the West African clade should be managed as UN3291 Regulated Medical Waste (RMW) in the same manner as other potentially infectious medical waste.
• Congo Basin Clade: Congo Basin clade is classified as Category A under the DOT hazardous materials regulations. See the DOT website for additional information.
Environmental infection control
Standard cleaning and disinfection procedures should be performed using an Environmental Protection Agency (EPA)-registered hospital-grade disinfectant with an emerging viral pathogen claim found on EPA’s List Q. Follow the manufacturer’s directions for concentration, contact time, and care and handling.
Soiled laundry should be gently and promptly contained in an appropriate laundry bag. It should not be shaken or otherwise handled in a manner that may disperse infectious particles.
Duration of precautions
Monkeypox can spread from the time symptoms start until the rash has fully healed and a fresh layer of skin has formed. Decisions regarding discontinuation of isolation precautions in a healthcare facility should be made in consultation with an infectious disease physician and the infection prevention and control department. Isolation precautions should be maintained until all lesions have crusted, those crusts have separated, and a fresh layer of healthy skin has formed underneath.
Visitation
Visitors to patients with Monkeypox should be limited to those essential for the patient’s care and wellbeing (e.g., parents of a child, spouse).
Margaret M. Miller, BS, MT(ASCP) M, CIC, FAPIC, is an infection preventionist with ECRI.
Susan Singh, MPH, CIC, is an infection preventionist with ECRI.
Resources:
Management of Patients with Suspected or Confirmed Monkeypox Virus. ECRI (pending publication). 2022
Monkeypox – Infection Control in Healthcare Settings. CDC. July 2022: https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
PA DOH fact sheet: https://www.health.pa.gov/topics/Documents/Diseases%20and%20Conditions/Monkeypox.pdf
Monitoring Persons Exposed. CDC. June 2022: https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
The Role of Environmental Hygiene in the Prevention and Control of Candida auris Transmission
By Susan Singh, MPH CIC
This article originally appeared in the July 2022 issue of Healthcare Hygiene magazine.
Candida auris (C. auris) is a serious public health threat. First identified in Japan in 2009, it is a multidrug resistant fungus which has caused outbreaks in healthcare facilities. Infections caused by C. auris often do not respond to commonly used antifungal drugs, making them difficult to treat and leading to severe infections and high mortality.
Many healthcare facilities around the world have reported surges of C. auris cases during the COVID -19 pandemic, most likely because of competing priorities and changes in infection prevention and control (IPC) practices during the pandemic. Further compounding these contributing factors to C. auris transmission, C. auris persists in the healthcare environment on various fomites and surfaces for weeks. Several studies have looked at the survival range for C. auris on various surfaces (Figure 1), suggesting that contaminated surfaces may be an important source of acquisition. C. auris frequently forms biofilms on surfaces. Research has shown that C. auris can survive on both wet and dry biofilms, making it harder to eradicate from the environment once it has been introduced.
Figure 1
| Material | Survival Range |
| Glass | 3 days |
| Plastics | > 14 days |
| Steel | > 7 days |
The fundamental element of cleaning to prevent transmission is highlighted in one outbreak investigation in the neurosciences intensive care unit at a hospital in the United Kingdom with a cluster of C. auris cases. Between Feb. 2, 2015, and Aug. 31, 2017, the unit identified 70 patients either colonized or infected with C. auris. The authors found an association of C. auris positivity with patients who had skin-surface axillary temperature monitoring via use of reusable probes. As the presumed source of C. auris, all temperature probes were removed from use. However, probe use was reestablished during the annual leave of a senior nurse, and C. auris acquisition continued. All probes were completely removed and cultured —C. auris was isolated from four of five probes presumed to be recently used, while none of the five probes in storage grew C. auris.
Beyond proper cleaning and disinfection, C. auris prevention requires other IPC practices. In July 2020, a Florida acute-care hospital faced an outbreak of C. auris on its specialty care unit dedicated to COVID-19 patients. A cluster of three C. auris bloodstream infections and one urinary tract infection prompted a joint outbreak investigation by the Florida Department of Health and the Centers for Disease Control and Prevention (CDC). Various breaches were identified including healthcare personnel (HCP) using multiple gown and glove layers, extended use of the underlayer of personal protective equipment (PPE), gaps in cleaning and disinfection of shared medical equipment/environment, and low hand hygiene compliance. After implementing enhanced cleaning and disinfection practices, removing supplies from hallways, and discontinuing base PPE layer practices, C. auris transmission halted.
If C. auris has been identified at your facility, here are some examples of steps you can take to ensure a safe environment for patients/residents, healthcare workers, and visitors:
- Disinfectant selection and contact time: The disinfectant used for patient-care equipment and/or the environment should be listed on the Environmental Protection Agency (EPA)’s List P: Antimicrobial Products Registered with EPA for Claims Against auris. It is also imperative that the contact time for the disinfectant selected is strictly followed. If the surface being disinfected dries before the contact time, the surface must be wiped down again.
- Reusable patient care equipment:
- If possible, dedicate reusable patient-care equipment for each auris patient (i.e., bladder scanner, vital signs machine, stethoscope).
- Observe the workflow of HCP to confirm “dirty” equipment is not brought outside the room (i.e., “dirty” glucometer machine being placed on the isolation cart outside patient room while the HCP doffs PPE inside the patient room). Develop a workflow to ensure contamination does not occur inside or outside the room.
- Audits: Initiate and/or increase the number of hand hygiene, PPE, and cleaning/disinfection audits. Provide real-time feedback and correction to non-compliant observations.
- Cleaning and disinfection responsibilities: Ensure all equipment is assigned an owner responsible for cleaning and disinfection. For example, identify if nursing or environmental services is responsible for disinfecting medication pumps during a hospital stay. At terminal cleaning a medication pump may be sent to the sterile processing department for cleaning and disinfection.
- Cohorting:
- Consider cohorting infected and/or colonized patients to one side of the unit or floor –avoid having a C. auris negative patient surrounded by two auris patients
- If feasible, consider cohorting staff
- Procedural areas:
- Confirm procedural areas are made aware when they are receiving a auris positive patient and know the appropriate PPE to wear
- When possible, C. auris patients should be scheduled for the last case of the day to allow for thorough terminal cleaning
- Supplies: Minimize the number of supplies/carts in the hallway which may easily be contaminated. Efforts must be made to take in all supplies needed for patient care upon initial entry into a auris positive patient room, thereby, minimizing the potential to contaminate supply carts.
Since its emergence more than a decade ago, the spread of C. auris across the world remains unabated. As we continue to see cases, we must remain vigilant in our efforts to mitigate C. auris transmission with strict adherence to IPC policies and procedures.
Susan Singh, MPH CIC, is an infection preventionist with ECRI.
References:
Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, Griffiths D, George S, Butcher L, Morgan M, Newnham R, Sunderland M, Clarke T, Foster D, Hoffman P, Borman AM, Johnson EM, Moore G, Brown CS, Walker AS, Peto TEA, Crook DW, Jeffery KJM. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N Engl J Med. 2018 Oct 4;379(14):1322-1331. doi: 10.1056/NEJMoa1714373. PMID: 30281988.
Morbidity and Mortality Weekly Report (MMWR) Candida auris Outbreak in a COVID-19 Specialty Care Unit — Florida, July–August 2020 Weekly / January 15, 2021 / 70(2);56-57.
Schelenz, S., Hagen, F., Rhodes, J.L. et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5, 35 (2016). https://doi.org/10.1186/s13756-016-0132-5
Smith J, et al. 2019 Clinical Microbiology Reviews.
Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, Litvintseva AP. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996-3005.
Widmann JE, Kirchhoff L, Brüggemann Y, Todt D, Steinmann J, Steinmann E. Persistence of Pathogens on Inanimate Surfaces: A Narrative Review. Microorganisms. 2021 Feb 9;9(2):343. doi: 10.3390/microorganisms9020343. PMID: 33572303; PMCID: PMC7916105.
How Healthcare Organizations Can Better Manage Commercially Prepared Sterile Supplies
By Diane Cullen, MSN, MBA, RN, CIC
This column originally appeared in the June 2022 issue of Healthcare Hygiene magazine.
Managing commercially prepared sterile supplies is an essential task and can be challenging for many healthcare organizations. As part of their effort, healthcare organizations must identify optimal locations within their facilities to store supplies so that staff can readily access them. Healthcare organizations must ensure supplies are being stocked to peak levels while keeping alert to expiration dates so that items do not expire. Additionally, storage areas must be properly maintained so that supplies are stored safely and kept in good condition while protecting them from contamination.
The Joint Commission receives multiple inquiries every week related to sterile supply storage concerns. By recognizing some of the following practices, healthcare organizations can better understand The Joint Commission’s expectations for managing and storing packaged sterile supplies within healthcare facilities.
Device Labeling
According to the Food and Drug Administration (FDA), manufacturers of certain medical devices and products must include labeling on or within their devices. ‘Labeling’ includes all actual labels and other written, printed, or graphic information located on equipment, devices, or wrappers. This includes but is not limited to instructions for use (IFU). Additionally, The Joint Commission requires that healthcare organizations follow manufacturer’s written instructions for use (IFU) to ensure the end-user understands how to properly use, clean, disinfect, reprocess, and store medical devices.
Below are six criteria manufacturers generally include in device labeling:
1. Reflect the intended use of the device (e.g., single use, single patient use)
2. Advise the end-user on how to thoroughly clean the device
3. Indicate the correct microbiocidal process (e.g., sterilization, high, intermediate, or low- level disinfection) for the device based on intended use
4. Include technically feasible Instructions
5. Provide comprehensive Instructions
6. Offer understandable Instructions
Device Labels
In 2016, the FDA published a final rule (21 CRF Parts 660, 801 and 809) which revised its medical device labeling regulations to allow for optional inclusion of symbols in labeling without additional explanatory text (e.g., ‘stand-alone symbols) if certain criteria were met. A device label may include important symbols that help end users easily understand key information about an item at a glance. For example, many commercially prepared sterile devices will include a manufactured date (the date the item was manufactured) which should not be confused with the expiration date (the date the item may no longer be used).
A single-use device intended to be used on an individual patient during a single procedure and then discarded may be represented by the number “2” with a horizontal slash across. Conversely, a medical device intended for a single-patient use, meaning it may be used on one patient but may be reprocessed between use per IFU is indicated by a completely different symbol. Another symbol with infection prevention implications on device packaging is the symbol for ‘Do not re-sterilize,’ which indicates the sterile medical device within the package must not be re-sterilized after being sterilized once. Additionally, the label may also include symbols that indicate temperature and humidity requirements for storage.
Included below are examples of common symbols that may be included on your packaged sterile products and their meanings.
Applying the Hierarchical Approach to Infection Prevention to Packaged Sterile Supplies and Devices:
The Joint Commission receives many questions about whether temperature and humidity monitoring are required for packaged sterile devices and instruments. It is important to follow the hierarchy when developing your practices around storage of these products.
Rules and Regulations
The first level of the hierarchy is that a healthcare organization ensures it is compliant with all building code requirements. Deemed organizations must fulfill Centers for Medicare and Medicaid Services (CMS) ventilation requirements which outline criteria for new or renovated existing facilities (constructed or plans approved on or after July 5, 2016). These are provided in the 2012 edition of NFPA 99 which references the 2008 edition of ASHRAE 170 table 7.1. If a local authority has published building codes, then a healthcare organization must meet the most restrictive requirement.
ASHRAE Standard 170- 2008 Table 7.1 ventilation requirements for sterile storage in CENTRAL MEDICAL AND SURGICAL SUPPLY areas. If an organization is storing sterile items in a room designated as a Central Medical and Surgical Supply Area, the following will be required:
• Positive air pressure relationship to adjacent areas
• Minimum outdoor air exchange two per hour
• Minimum total air exchange four per hour
• Maximum relative humidity of 60 percent
• Temperature range 72 to 78 F or 22 to 26 C
CMS Requirements
The CMS Infection Control Worksheet for the Hospital (HAP) and Ambulatory Surgical Center (ASC) accreditation programs is one of CMS’ requirements. Depending on the type of facility surveyed, these organizations must meet Conditions of Participation (CoP) or Conditions for Coverage (CfC). CMS requires that sterile packages are stored so that sterility is not compromised, and sterile items are inspected for integrity before use.
Manufacturer’s Instructions for Use
Organizations must be compliant with the manufacturer's instructions for storage as indicated on the label. If, for example, the manufacturer of the sterile supply requires a specific temperature and humidity requirement for storage, an organization would need to demonstrate during survey that these requirements are being met. The Joint Commission does not specifically require that these parameters be documented, however a facility’s staff should be able to identify if any sterilized supply, has been potentially compromised (as may occur if the integrity of the package is in question or has evidence of damage from humidity) and should be able to determine whether that item would be appropriate for use.
Evidence-Based Guidelines and National Standards
Health care organizations may refer to evidence-based guidelines and national standards (EBGs) for guidance for how sterile supplies should be stored. Most EBGs agree that sterile supply areas must be clean, well ventilated and should protect supplies from contamination, moisture, dust, temperature extremes, and humidity extremes. Healthcare organizations must show evidence that, whether in a designated central surgical supply area or in a storage room with mixed clean and sterile supplies, storage techniques are protecting supplies from contamination and maintain the integrity of the packaging from damage. Failure to store medical and sterile supplies in a manner to protect from contamination will be scored according to Standard IC.02.02.01 EP 4.
For more information on how healthcare organizations can better manage commercially prepared sterile supplies, please contact The Joint Commission’s Standards Interpretation FAQ Page to receive answers to specific questions.
Diane Cullen, MSN, MBA, RN, CIC, is assistant director of standards interpretation at The Joint Commission.
References:
https://www.fda.gov/medical-devices/unique-device-identification-system-udi-system/udi-basics
https://www.fda.gov/medical-devices/device-labeling/quality-system-regulation-labeling-requirements
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/labeling-regulatory-requirements-medical-devices-fda-89-4203
https://www.fda.gov/news-events/fda-voices/using-symbols-convey-information-medical-device-labeling
https://www.jointcommission.org/standards/standard-faqs/ambulatory/environment-of-care-ec/000001275/
How Can We Connect the Dots Between a Missed Hand Hygiene Opportunity and Subsequent Patient Harm?
By Barbara DeBaun, MSN, RN, CIC
This column originally appeared in the May 2022 issue of Healthcare Hygiene magazine.
It’s been 175 years since Ignaz Semmelweis proposed the practice of washing hands with chlorinated lime solution as a strategy to prevent transmission of potentially lethal bacteria. The Centers for Disease Control and Prevention (CDC) issued its first guideline for handwashing in 1985 and the APIC guidelines followed 10 years later. Guidelines have been updated over the years with new knowledge and alternative approaches to hand hygiene such as alcohol-based hand sanitizer. The Joint Commission issued the first set of National Patient Safety Goals in 2002 and, not surprising, one of the goals was to reduce the risk of healthcare associated infections by complying with either the current CDC hand hygiene guidelines or the current World Health Organization (WHO) hand hygiene guidelines.
Infection preventionists are leading efforts worldwide to impact hand hygiene compliance in healthcare facilities. We oversee the hand hygiene observation programs, recruit observers, train them, analyze their findings, display data, and report findings to multiple committees and stakeholders. We conduct activities using black light technology to ‘make the invisible germs visible’ and do our best to find ways for fun and interactive activities. We find ways to provide reward and recognition when observing a good example of hand hygiene with every ounce of creativity. Some of us have established code phrases such as “Gel-in, gel out,” “Can I give you a hand?” “Dr. Hand is on the phone,” or even “Touchdown” for an observed hand hygiene and “Fumble” when observing a missed opportunity. These code phrases can be quite effective, but they might be brushed off or dismissed as a subtle suggestion, rather than a hard stop.
So, let’s shift gears for a moment. Think about all the safety checks we have in place to minimize the chance of a patient receiving the wrong type of blood or the wrong medication. It is estimated that in the United States alone, between 7,000 and 9,000 people die every year because of a medication error. Approximately 20 people in the U.S. die every year after receiving an incompatible transfusion of blood. In most circumstances, administration of incompatible blood results in an immediate and dramatically obvious error. The nurse who hung the blood will see the impact of the error quickly and connect the dots between the wrong blood and the patient harm. If a nurse administers a medication to a patient with a severe allergy or grabs the wrong concentration or heparin and delivers a much higher dose than indicated, the error will be obvious. The dots between the human error and the harm to the patient get connected.
But what happens if a healthcare provider fails to perform hand hygiene and touches a patient’s central line with contaminated hands? Might the patient develop a central line-associated bloodstream infection (CLABSI)? Very possibly. But the difference is the healthcare-associated infection will not be obvious immediately, such as with a blood transfusion reaction or a severe systemic allergic reaction. Even though the healthcare provider’s contaminated hands are a possible source of the CLABSI, the time delay between the breach and onset of infection is significant, therefore the dots don’t get connected.
So, how can we change the conversation and get better at not only seeing a hand hygiene failure as a human error, but to create a powerful peer-to-peer support system where staff welcome and appreciate a direct reminder to do the right thing for patient safety?
You might want to try some role playing that goes something like this. Approach a nurse and ask the following:
1. If I saw you about to hang the wrong blood on your patient, would you want me to stop you?
2. If I saw you about to give penicillin to a patient with a severe penicillin allergy, would you want me to stop you?
See how the nurse responds, but likely s/he will say “Yes” or “Absolutely” without missing a beat.
Next, ask the nurse:
1. If I saw you about to touch a patient without washing your hands, would you want me to stop you?
The response might be a little different. Some will respond with an immediate “Yes,” and others might hesitate a bit because this particular question may feel more personal or judgmental. The important outcome of this exercise is to create a dialogue and have the conversation that promotes ‘connecting the dots’ between a human error and patient harm.
Ultimately, we want our healthcare providers to view healthcare associated infections as preventable harms that are just as significant as those that result from medication or blood administration errors.
Go get those dots connected!
Barbara DeBaun, MSN, RN, CIC, is improvement advisor for Cynosure Health.
Best Practices in Sterile Processing to Reduce Surgical Site Infections
By Gail Horvath, MSN, RN, CNOR, CRCST, and Margaret Miller, BS, MT(ASCP) M, CIC, FAPIC
This column originally appeared in the April 2022 issue of Healthcare Hygiene magazine.
What are the most common contributing factors to surgical site infections (SSIs) in your organization? Certainly, sterile processing practices would make the list. It is reported that SSIs account for 20 percent of all hospital-acquired infections and that half may be preventable with implementation of evidence-based strategies.1 Having a robust sterile processing department (SPD) creates a strong first link in the chain of infection prevention, especially utilizing the following best practices.
Point of use care of instruments
Care and cleaning of surgical instruments is a shared responsibility between the SPD and the area where the instruments are used. Gross soil should be removed at the point of use to:
• Reduce the number of microorganisms on the item
• Reduce the nutrient material that might support microbial growth
• Reduce the potential for environmental contamination by aerosolization or spillage
• Minimize damage to devices from such substances as blood, saline, iodine, and radiological dyes.
Transport of instruments
Establish and implement standardized sterile processing procedures and measures for oversight of all aspects of processing and transport, including when transporting between facilities. When transporting instruments to the operating room (OR)/procedure area, contain sterile items in a closed system to protect items from contamination, damage, or tampering. When returning instruments to SPD post-procedure, contain contaminated items in a closed system and transport to the decontamination area as soon as possible.
If transporting instruments off-site, the facility must comply with applicable Department of Transportation and state regulations. Clean and contaminated items should be separated to prevent cross-contamination during transport. Transport vehicles that are loaded and ready for transport should not be left unattended in unsecured areas.
Instrument inspection
In SPD, use lighted magnification to inspect instruments. Test insulated equipment for current leakage
and remove defective instruments from service. Instruments should be thoroughly dry before assembly
and packaging.
In the OR, confirm the following prior to placing instruments on the sterile field:
• Integrators have turned
• Biological for the load was negative
• Integrity of the container was not compromised
• No holes were present in wrapped trays
• No visual bioburden or debris was present
Loaner instruments
Ensure vendors provide manufacturer instructions for use (IFUs) for instruments and that they are delivered with adequate time for onsite decontamination and sterilization prior to the scheduled case.
Water and steam quality and monitoring
Water quality is an important consideration in all stages of medical device reprocessing. Poor water and steam quality may damage surgical instruments. It is best practice to use critical water—water that is extensively treated by a multistep treatment process that usually includes a carbon bed, softening, deionization and/or reverse osmosis—for the final decontamination rinse and for steam generation. Routinely monitor water quality (bacteria, pH, chloride, hardness, conductivity, endotoxins) and share the results with infection prevention and control as well as SPD leadership.
Immediate use steam sterilization (IUSS)
Formerly called “flash sterilization,” IUSS is described by The Joint Commission as "the shortest possible time from the item being removed from the sterilizer to the aseptic transfer onto the sterile field." IUSS items are not intended to be stored for future use.
Acceptable conditions to use IUSS:
• The item will be used immediately
• Terminal sterilization is not an option
• The device manufacturer's written instructions for cleaning, cycle type, exposure times, temperature settings, and drying times (if recommended) are readily available and followed; and include instructions for IUSS
• The IUSS rigid sterilization container manufacturer's written IFUs are followed
• IUSS should not be used for mere convenience, or due to limited instruments or equipment for the number of cases/procedures performed.
Event-related sterility
Event-related sterility is based on the premise that items that have been properly decontaminated, cleaned, wrapped or containerized, stored, and handled will remain sterile indefinitely unless opened.
However, events that may affect the sterility of a package include, but are not limited to:
• Excessive handling that leads to seal breakage or loss of package integrity
• Compression during storage
• Moisture penetration
• Exposure to airborne or environmental contaminants
• Storage conditions where temperature is in excess of 75 degrees F (24 degrees C) and/or humidity is in excess
of 70 percent
• Packages are dropped
• Missing external indicator of sterility (e.g., autoclave tape)
• Internal integrators have not changed.
Environmental cleaning in sterile processing areas
Before work begins for the day, damp dust from top to bottom (sterilizers, workstations, counters, shelving). Daily terminal cleaning includes floors in all areas, workstations, sinks, pass-through window, and trash and linen receptacles.
In summary, SSIs are among the most preventable healthcare-associated infections and are a significant burden in patient morbidity, mortality, and costs. To ensure quality, we suggest auditing of key areas, such as IUSS rates, and if loaner instruments and implants are delivered soon enough for onsite decontamination and sterilization. Lastly, we recommend reviewing the recently published ANSI/AAMI ST91: 2021 Flexible and semi-rigid endoscope processing in health care facilities. This revision provides expanded guidance on point of use treatment, transport, leak testing, cleaning, high-level disinfection, liquid chemical sterilization, packaging, sterilization, and storage of flexible and semi-rigid endoscopes to ensure endoscopes are safe for patient use.
Gail Horvath, MSN, RN, CNOR, CRCST, is senior patient safety analyst and consultant with ECRI.
Margaret Miller, BS, MT(ASCP) M, CIC, FAPIC, is an infection preventionist with ECRI.
References:
1. Ban K, et al. American College of Surgeons/Surgical Infection Society: surgical site infection guidelines, 2016 update.
National Healthcare Safety Network Surgical Site Infection Event. CDC. 2022.
Societies Call for Significant Need to Increase Capacity Across the U.S. Healthcare System
By HHM staff
This column originally appeared in the March 2022 issue of Healthcare Hygiene magazine.
As the world enters the third year of the COVID-19 pandemic, the Association for Professionals in Infection Control and Epidemiology (APIC) is issuing an urgent call-to-action to shore up the nation’s infection prevention and control (IP&C) infrastructure.
Even before the pandemic, hospital IPC programs were underfunded and understaffed. The pandemic exacerbated those patient safety weaknesses, leaving healthcare facilities with insufficient capacity to prevent common, often deadly, healthcare-associated infections.
Published in February, APIC’s Between a Rock and a Hard Place: Recommendations for Balancing Patient Safety and Pandemic Response, provides an extensive set of strategies to increase the IP&C workforce, strengthen prevention programs, and build resiliency for future pandemics.
“APIC is issuing this call-to-action as we all recall the nightmare of extensive supply shortages and overworked healthcare workers,” says 2022 APIC president Linda Dickey, RN, MPH, CIC, FAPIC. “Especially troubling to APIC is how many preventable infections were transmitted inside hospitals during COVID because that resilience was not built into our healthcare system.”
In the report, APIC urges policymakers to allocate funding to build IPC surge capacity to ensure the continuity of safe patient care during a pandemic. The specific recommendations from the 66-page report include:
· Develop next-generation universal personal protection equipment (PPE) for a one-size-fits-all device to protect healthcare workers
· Normalize the use of masks by the public during outbreaks of infectious diseases, building trust among the American people of their effectiveness
· Address supply chain failures to ensure greater diversity in production locations and expanded ease of access
· Require that healthcare facilities include personnel with IP&C expertise on emergency response teams to ensure the safety of response practices
· Protect nursing home residents ensuring that each nursing home has at least one dedicated infection prevention expert on staff
· Build and implement IP&C surge capacity to ensure the continuity of safe patient care during a pandemic
· Increase capacity for testing and contact tracing to control disease spread during a pandemic
· Ensure rapid healthcare data collection and sharing to optimize strategies to prevent disease transmission
· Build vaccine confidence to combat misinformation and dissuade hesitancy
· Fund pandemic preparedness workforce capacity and training with incentives for universities to create a pathway to the infection prevention profession
In 2021 the Centers for Disease Control and Prevention (CDC) documented a sharp rise in healthcare-associated infections (HAIs), which had been steadily decreasing prior to the pandemic.
Because of the strain that the pandemic put on the entire healthcare system, central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), ventilator-associated events (VAE), and methicillin-resistant Staphylococcus aureus (MRSA) have increased exponentially.
After years of steady reductions in healthcare-associated infections, significantly higher rates of four out of six routinely tracked infections were observed in U.S. hospitals, according to a CDC analysis of data from the National Healthcare Safety Network (NHSN) published in Infection Control & Hospital Epidemiology. Increases were attributed to factors related to the COVID-19 pandemic, including more and sicker patients requiring more frequent and longer use of catheters and ventilators as well as staffing and supply challenges.
“COVID-19 created a perfect storm for antibiotic resistance and healthcare-associated infections in healthcare settings. Prior to the pandemic, public health — in partnership with hospitals — successfully drove down these infections for several years across U.S. hospitals,” said Arjun Srinivasan, MD, CDC’s associate director of Healthcare Associated Infection Prevention Programs. “Strengthening infection prevention and control capacities works. This information emphasizes the importance of building stronger, deeper and broader infection control resources throughout healthcare that will not only improve our ability to protect patients in future pandemics but will also improve patient care every day.”
For this analysis, researchers used data collected through NHSN, the nation’s largest healthcare-associated infection surveillance system, which is used by nearly all U.S. hospitals to fulfill local, state, or federal infection reporting requirements. Major increases were found in 2020 compared to 2019 in four serious infection types: central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated events, and antibiotic resistant staph infections. The largest increases were bloodstream infections associated with central line catheters that are inserted into large blood vessels to provide medication and other fluids over long periods. Rates of central line infections were 46 percent to 47 percent higher in the third and fourth quarters of 2020 compared to 2019.
With dramatic increases in the frequency and duration of ventilator use, rates of ventilator-associated infections increase by 45 percent in the fourth quarter of 2020 compared to 2019. The CDC analysis found sharp increases in standardized infection rates, indicating that the increases were not simply a reflection of more devices being used.
“Infection control practices in COVID-19 wards often adapted to shortages of personal protective equipment, responded to fear of healthcare personnel, and did not always lend themselves to better infection prevention,” said Tara N. Palmore, MD, and David K. Henderson, MD, of the National Institutes of Health, in an editorial that accompanied the study. “The success of the previous several years, with steady declines in rates of these (healthcare-associated) and device-related infections, further accentuated the upswings that occurred in 2020.”
The study found that two other types of infection remained steady or declined during COVID-19. Surgical-site infections rates did not increase as fewer elective surgeries were performed, largely in operating rooms with uninterrupted infection control processes that were separate from COVID wards. In addition, no increase was found in Clostridioides difficile. The study said lower rates of C. diff may be a result of increased focus on hand hygiene, environmental cleaning, patient isolation, and use of personal protective equipment.
“Basic infection control practices must be hardwired into practice so that they are less vulnerable when the health care system is stressed,” the editorial concluded, “One approach might be to designate clinical staff to be added to the hospital epidemiology team to allow for rapid expansion of effort to support a pandemic response.”
At the time this study was published in August 2021, APIC then-president Ann Marie Pettis, BSN, RN, CIC, FAPIC, had called this CDC data “quite troubling and must serve as a call to action.” She added, “As a nation we must take significant efforts to bolster our infection prevention and control programs throughout the healthcare continuum. The report highlights the need for healthcare facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combatting HAIs are not lost.”
Pettis continued, “The unfortunate reality is that in one year we lost nearly a decade of progress against HAIs like central line-associated bloodstream infections, catheter-associated urinary tract infections, MRSA, and ventilator-associated events. We now have an opportunity to use this data and take action to invest in our public health infrastructure, expand our nation’s infection prevention and control workforce, and put infection preventionists – specialists who are trained and certified to prevent infections — at the center of these efforts. APIC is calling on healthcare facilities to assess their infection prevention programs by looking at the care and services they provide and determining the appropriate level of personnel and resources necessary to protect patients and healthcare workers. Facility-wide infection prevention programs are critical and require adequately staffed, trained, and resourced infection prevention and control departments. APIC also calls on federal and state governments to provide funding to help support healthcare facilities across the continuum of care to ensure that there is adequate surge capacity so that infection prevention and control measures will endure when stressed by future pandemics and disease outbreaks.”
As we know, HAIs can often be prevented through careful monitoring and safety protocols overseen by infection preventionists, but only when there are sufficient resources and trained personnel in place to support these efforts.
“For the U.S. to create a safer, more resilient healthcare system, policymakers should make the substantial investments recommended by the hands-on infection prevention experts who had a unique vantage point as the pandemic overwhelmed hospitals, nursing homes and clinics nationwide,” says Dickey.
“We need to build capacity so we can surge when we need to,” says APIC’s CEO Devin Jopp, EdD, MS. “I won’t sugarcoat it; fortifying our nation’s IPC infrastructure isn’t free, but the cost of ill-preparedness in lives and dollars is incalculable.”
To help healthcare facilities assess their IP&C capacity, APIC is launching a new campaign called HAI Fast Forward: Accelerating HAI Prevention. It will include a series of initiatives to help organizations make headway in reducing their HAIs back to pre-pandemic levels.
Other organizations are responding to the call for addressing post-pandemic capacity needs. The Society for Healthcare Epidemiology of America (SHEA) says it agrees with the call to rebuild a resilient patient safety culture in U.S. healthcare institutions that came in late February from the Centers for Disease Control and Prevention (CDC) and the Centers for Medicare and Medicaid Services in The New England Journal of Medicine.
As SHEA explains, "The pandemic response has led to burnout and staffing shortages, which have compounded the challenges associated with maintaining culture of safety in healthcare settings. Addressing these realities and creating a patient safety culture that values the critical role of healthcare personnel is essential to building a stronger future for healthcare-associated infection prevention. We are now faced with a pivotal opportunity for healthcare leaders to take lessons learned from the COVID-19 pandemic to rebuild better systems of care for healthcare-associated infections that can be managed during outbreaks and other crises impacting healthcare.”
SHEA has been updating education and expert guidance to incorporate lessons learned from COVID-19 into the next generation of infection prevention and antibiotic stewardship programs. We look forward to working across government agencies, healthcare systems and patient advocacy groups to refocus on healthcare-associated infection prevention with an emphasis on more sustainable responses post-pandemic."
Also in a statement issued in February, APIC said it was calling for healthcare organizations to assess their infection prevention capacity and strengthen prevention programs by adding personnel, resources, and training to support both infection prevention and surge capacity for future pandemics.
In the statement, 2022 APIC president Linda Dickey, RN, MPH, CIC, FAPIC, had stated, “The CDC reports that healthcare-associated infections increased significantly during 2020, reversing years of progress. The current pandemic illustrates that our healthcare facilities are not where they need to be in terms of infection prevention and patient safety. Facility-wide infection prevention programs are critical and require adequately staffed, trained, and resourced infection prevention and control departments. We must bolster our infection prevention and control staff capacity in our system of healthcare to simultaneously manage HAIs and future pandemics.”
Dickey added, “This is not the time to ask infection prevention teams to do more with less. It is the time for investment in the infection prevention and control infrastructure in our nation’s healthcare facilities so that basic infection control practices can be hardwired into processes of care. Our hospitals need more infection preventionists. Infection preventionists serve as a critical line of defense in preventing and responding to infections and integrating evidence-based strategies to limit their spread. It’s essential that hospitals, clinics, and long-term care facilities have enough infection preventionists to train staff and monitor safety protocols so that dangerous pathogens do not spread and lead to infection."
She continued, “APIC calls on federal and state governments to provide funding to help support healthcare facilities across the continuum of care to ensure that there is adequate surge capacity so that infection prevention and control measures will endure when stressed by future pandemics and disease outbreaks. We can’t let the lessons learned from COVID-19 go to waste. Building stronger infection prevention programs throughout healthcare will not only improve our ability to protect the public during future pandemics but will simultaneously improve patient safety. To help healthcare facilities assess their infection prevention capacity, APIC is launching a new campaign called HAI Fast Forward: Accelerating HAI Prevention, which will include a series of webinars and other resources available to help organizations make headway in reducing their HAIs back to pre-pandemic levels.”
References:
Palmore TN and Henderson DK. Healthcare-Associated Infections in the Time of Pandemic COVID-19. Infection Control & Hospital Epidemiology. Web (Aug. 25, 2021).
Weiner-Lastinger LM, et al. The impact of COVID-19 on healthcare-associated infections in 2020; A summary of data reported to the National Healthcare Safety Network. Infection Control & Hospital Epidemiology. Web (Aug. 25, 2021).
Urine Culture Practices: Playing Better Defense
By Barbara DeBaun, MSN, RN, CIC
This column originally appeared in the February 2022 issue of Healthcare Hygiene magazine.
As an infection preventionist, it is not uncommon to review the medical record of a patient who developed a hospital-onset urinary tract infection and then ask the question, “why did they culture this?” We might see a urine culture result in a patient that had no obvious source of a urinary tract infection (UTI). Or one in a patient who was ‘pan-cultured’ when appearing septic even though the source of the sepsis was obviously something other than the urinary tract. It is also not unusual for a clinician to order a urine culture on a patient who is clearly at the end of life, yet all sources of fluids and body substances were cultured at the bitter end even though the results were not going to be acted upon.
Hospital electronic medical records have made it easy to order a urine culture. It can be as simple as clicking a box.
When a patient’s urine culture is reported as positive, it may be difficult for the clinician to ignore it. The clinician will weigh the risks and benefits of treating vs not treating. If the culture result suggests true infection, treatment is indicated. However, if the culture is interpreted as positive despite the patient lacking symptoms of a UTI, the patient will receive unnecessary antibiotics. We all know how that can end.
Culturing patients that do not have clinical symptoms or a potential to be treated for a urinary tract infection is an avoidable medical error. Unnecessary treatment with antibiotics harms patients. It can result in drug-drug interactions, C. difficile infection, multidrug-resistant bacteria, renal damage, allergies, increased length of stay and other complications.
Multiple myths have perpetuated the knee-jerk ordering of urine cultures in the absence of clinical indications. Some myth-busting facts are as follows:
- An abnormal urinalysis does not necessarily indicate a UTI. Asymptomatic bacteriuria is quite common, and we also know that collection and transportation of urine specimens can be problematic when not done correctly or timely
- Smelly, cloudy urine is a common finding in patients who are dehydrated.
- Elderly or otherwise deconditioned patients can have asymptomatic bacteriuria, therefore symptoms such as weakness, fatigue or mental status changes are not necessarily a reason to suspect UTI
- Screening patients with NO symptoms of a UTI is a recipe for failure and patient harm. We should screen patients for dietary and hydration habits and address those issues before assuming the patient has a UTI when dehydration is much more likely to be the reason for the concentrated, smelly urine
How do we make it easier to do the right thing, and harder to do the wrong thing? Diagnostic stewardship or leveraging the clinical laboratory to improve antimicrobial stewardship is key. Our microbiology partners are highly trained to report what they see. If white blood cells are observed in the urinalysis, these will be reported. Unfortunately, white blood cells in the urine (pyuria) can’t differentiate asymptomatic bacteriuria from a true urinary tract infection. So, there is the conundrum.
Keys to success include:
- Ordering: Test urine only when clinical symptoms suggest a UTI or if testing syncs with current guidance to screen patients scheduled for urologic surgery or those who are pregnant
- Collection: Go to Gemba and observe how and why urine specimens are being collected; we often make assumptions about how things are done and there is no better way to know the truth than to go look.
- Processing: Determine criteria for advancing a urinalysis to culture based upon criteria established by your facility
A recent study by Dougherty, et al, reported the impact of a urine culture s standardization program that included order indications and urinalysis (U/A) with reflexive culture. The team determined that 64 percent of urine cultures ordered using the reflexive test did not reflex to culture by U/A criteria.
- Reporting: Provide clear guidance to clinicians to make it easier for them to make the best decision (e.g., to not treat a patient who has an unlikely probability of having a UTI). Clinicians want to do the right thing, but they are easily tempted to treat a patient because a positive test is hard to ignore.
So, perhaps we should think of diagnostic stewardship using a sports analogy. Soccer teams have five defensive players. The sweeper, fullback, center back and wing back are tasked with preventing the soccer ball from entering the goal (i.e., avoid sending urine specimens that don’t make clinical sense). The goalie is the person who can put a stop to the score when the other four defensive players can’t get the job done (i.e., prevent the processing and reporting of a urine culture that has a low probability of being ‘real’). Ideally, processes need to be in place to prevent urine specimens from being collected and sent to the laboratory in the first place. When they are clinically indicated, do it right. But we know that sometimes our defense fails us, and we need our goalie (i.e., urinalysis with reflexive culture) to prevent a score for the opposing team.
It takes a team whether it’s a sport or healthcare, so explore strategies for practice change that make it easy to do the right thing, and hard to do the wrong thing.
Barbara DeBaun, MSN, RN, CIC, is improvement advisor for Cynosure Health, where she provides vision and leadership in the development, implementation and facilitation of infection prevention and quality improvement initiatives for healthcare organizations. She has 40-plus years of experience in infection prevention and quality improvement.
Reference: Dougherty DF, Rickwa J, Guy D, Keesee K, Martin BJ, Smith J, Talbot TR.Reducing inappropriate urine cultures through a culture standardization program. Am J Infect Control 2020;48:656-662.
Ensuring Proper Selection of Disposable Medical Protective Gowns
By Karen Haberland
This column originally appeared in the January 2022 issue of Healthcare Hygiene magazine.
Medical protective gowns are intended to protect the wearer from potentially infectious solids, liquids, or airborne particulate. Gown wearers—which can include healthcare workers, non-clinical personnel, visitors, and others—can be put at risk of cross-contamination if the wrong type of gown is purchased and worn for the intended application, or if the gown does not provide the level of protection that it claims.
Selecting the appropriate gown for any given application, however, is not a simple task.
The type of gown worn depends on several factors, including the clinical role, patient diagnosis, required isolation level per hospital protocol, type of treatment, etc. For example, gowns intended for surgical purposes may focus a high degree of fluid protection in the front and arms of the gown, while a gown intended for isolation with a risk of infectious airborne particulate would need 360-degree coverage.
There is a vast array of options: from fluid impervious, full coverage surgical gowns to moderately protective isolation gowns to low protection cover gowns and many in-between. Standards define the criteria for some – but not all – gown types. The Advancement of Medical Instrumentation (AAMI)’s PB70 standard outlines specifications for isolation and surgical gown fluid resistance levels (see Table 1). ASTM International’s F3352 and F2407 also define additional specifications regarding textile strength and breathability for isolation and surgical gowns, respectively.
Unfortunately, these standards are considered voluntary and are not strictly enforced for most gowns. This has led to the establishment of so-called cover or protective gowns, which may or may not offer the amount of coverage required of an isolation gown and may not be tested for fluid barrier performance. While these may offer sufficient protection for many activities, in-house review is necessary to ensure material strength and fluid barrier protection is equal to the task.
Furthermore, the nomenclature used by gown suppliers to designate the gown type or protection level is not consistent. Terms may be used interchangeably, or in a manner that does not align with how they are defined in the standards. For example, product literature may describe a product as an "isolation gown" even though it does not meet the AAMI PB70 requirements for isolation gowns, such as providing 360° coverage from neck to knees and at least minimal resistance to water spray (AAMI Level 1). The imprecise use of terms in marketing literature and lack of detailed specifications can make it difficult for purchasers to know the level of barrier protection that a gown will provide.
In a review of top-selling disposable gowns, ECRI has also noted that the marketing literature for many lack fluid barrier level claims, or claim a product is an isolation gown when it offers no back coverage. There is concern that these popular gowns may be worn in a higher risk environment than they can withstand, as they offer less than full coverage and unknown protectiveness. Thus, ECRI has recently begun to test a small sample of disposable isolation gowns. Preliminary findings suggest that some may not meet their claimed AAMI fluid barrier level. We hope to publish our findings in early 2022.
ECRI recommends that facilities gain a greater understanding of the properties of the various types of gowns, and stock the right gown for each procedure based in the risk of exposure. Remember that most gowns are not fluid impervious and are not tested against blood or bodily fluids. Also note the risk for airborne contaminants or the likelihood that the user may bump into equipment or turn his or her back on a patient, which may necessitate a full coverage gown over one with an open back.
There is no “one gown” that works for all a facility’s needs. Consider the following when selecting each type of gown:
• Disposable or Reusable
o Perform a spend analysis to determine which option is more economical for your facility.
o Consider storage area. Laundered reusable gowns require more space.
o Review the number of allowable washing and/or drying cycles. Manufacturers must test that barrier protectiveness does not degrade over time. Facilities must also plan for tracking and removal of old gowns.
o Reusable materials may be more durable for long-term use but may also be less breathable and hence uncomfortable to wear for long periods of time.
• Coverage
o Full coverage is necessary for activities with a risk of airborne contamination.
o Partial coverage may be sufficient for lower-risk tasks or those when the user’s back remains facing away from the patient.
• Fluid Barrier Level
o Choose the AAMI barrier protection level that best matches the exposure risk level of each procedure.
o If the manufacturer does not claim a fluid barrier level, use only for procedures with minimal risk of fluid exposure until tested for performance.
• Sizing
o One-size-fits-all is not adequate for all body types. Stock multiple sizes to ensure proper coverage without cumbersome excess for both petite and larger body types.
• Vetting Suppliers
o Fully vet all new suppliers prior to purchase. Consider whether they are new to the industry, or if they have a long history.
o Request product specifications and a list of standards to which the gowns comply.
o Request gown testing data to confirm barrier protectiveness levels.
o If possible, evaluate fluid barrier protectiveness prior to use.
• Proper Use
o Ensure personnel are trained in appropriate donning and doffing procedures, including properly securing both waist and neck fasteners to prevent the gown from gaping open.
o Remove gowns with care, per manufacturer’s instructions, to ensure clothing does not become contaminated.
While they may not garner as much attention as masks, gowns are a critical component of PPE and play an important role in reducing cross-contamination between patients and healthcare workers. It is crucial that facilities ensure staff is equipped with the knowledge and clothing to remain safe and healthy.
Karen Haberland, MS, is senior project officer, device evaluation, at ECRI.
References:
American National Standards Institute/Association for the Advancement of Medical Instrumentation (ANSI/AAMI). Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities. ANSI/AAMI PB70:2012.
ASTM International. Standard Specification for Isolation Gowns Intended for Use in Healthcare Facilities. ASTM F3352-2019.
ASTM International. Standard Specification for Surgical Gowns Intended for Use in Healthcare Facilities. ASTM F2407-20.
Kilinc Balci FS. Isolation gowns in health care settings: laboratory studies, regulations and standards, and potential barriers of gown selection and use. Am J Infect Control 2016 Jan;44(1):104-11. Available from: https://www.sciencedirect.com/science/article/pii/S0196655315008470?via%3Dihub#bib81.
ECRI. Medical Protective Gowns: Key Features and Guidance for Selection [ECRI Guidance]. Device Evaluation 2021 Aug 8. Available from: https://www.ecri.org/components/HDJournal/Pages/Medical-Protective-Gowns-Guidance-for-Selection.aspx.
Use of isolation gowns purchased from non-traditional manufacturers without independent lab validation may put healthcare workers at risk for blood and fluid exposure [ECRI Exclusive Hazard Report]. Alerts Guide 2020 Nov 5. Accession No. H0650. Available from: https://alerts.ecri.org/alerts-search/view/details/1644891.
Keeping Instruments Appropriate for Reprocessing
By Sylvia Garcia-Houchins, RN, MBA, CIC
Editor's note: This column originally appeared in the December 2021 issue of Healthcare Hygiene magazine.
In 2018 approximately 14.4 million operative procedures took place during inpatient stays1 and an estimated 129 million outpatient surgical procedures were performed.2 Add that information to results from an American College of Surgeons study reporting that 5.9 percent of trays delivered to the operating room (OR) contain broken instruments, and the statistics are staggering. It means that during that single year, more than 8 million OR trays potentially contained at least one broken instrument.
The Joint Commission’s infection prevention and control department focuses its efforts on the cleanliness of instruments since we have been taught that, “if it isn’t clean, it isn’t sterile.”3 If there is tissue, bone, or other soil left behind from a prior surgery, the instrument’s unsterile condition could carry risk of infection. The Joint Commission does not just focus on cleanliness of instruments when tracing sterilized instruments. Our surveyors also are trained to determine if an item is appropriate for reprocessing. Since potential exists for organizational liability related to surgical instruments that should not be reused, part of The Joint Commission survey process is to work with customers to identify these potential areas of liability. There are a variety of reasons that surgical instruments are not appropriate for reprocessing including but not limited to: pitting, oxidation, cracks, damaged insulation, or connectors, flaking and sticky tape, and bioburden. ECRI noted that 34 percent of soiled instruments resulted from inadequate cleaning – a violation that should be caught during the instrument inspection process prior to packaging – not at point of use.
In addition to instruments in disrepair making their way to the sterile field, Joint Commission surveyors have noted with increasing frequency the reprocessing of single-use devices (SUDs) – items labeled by their manufacturer as “single-use” or disposable. These devices are often made of lower quality materiel and as a result, develop pitting and oxidation which indicates to the surveyor that something might be amiss. SUD reprocessors are regulated by the Food and Drug Administration (FDA) and are subject to all the regulatory requirements currently applicable to the original device manufacturer, including premarket submission requirements. If a healthcare organization reprocesses a single use device, it is dangerously allowing itself to become a manufacturer of a medical device – and thus assuming all the manufacturer’s liability should that device fail. In addition, it takes on the liability of possible FDA rule violation.
Healthcare organizations follow key measures to ensure that their instruments are appropriate for reprocessing. These include:
• Before reprocessing any device, obtain the manufacturer’s validated reprocessing instructions. Review to ensure they meet the intended level of reprocessing based on intended use of the item and to ensure your organization has the appropriate equipment and products to follow the instructions.
• Ensure staff who are performing inspection are competent and able to perform the inspection process. Ensure staff knows which equipment dismantles and how to inspect it. Healthcare organization leadership should provide staff with good lighting and tools such as lighted magnifiers and other helpful equipment to ensure ample vision while inspecting.
• Empower staff to identify instruments of subpar quality and prevent their use – even if it means delaying or rescheduling affected cases.
• Establish effective maintenance and refurbishment processes to keep instruments in optimal condition. Determine when to remove an instrument that is no longer safe to undergo reprocessing.
• Ensure staff at point-of-use can identify when an instrument should not be used – even if it is in sterile packaging- and how to return and report the occurrence for quality monitoring.
• Train users to appropriately discard SUDs and support the process by ensuring disposal containers at point of use for safe disposal.
• Train users on key issues that can lead to damage of instruments and devices, including erroneously using delicate instruments meant for tissues on other items (e.g., gauze, tape, tubing, etc.), prolonged exposure to blood and other body fluids, or allowing these substances to dry on instruments, use of saline or corrosives such as bleach or inappropriate cleaning chemicals, use of abrasives, or transporting instruments in a way that places them at risk of damage.
• Sometimes instruments are damaged during use. Create and effective process for identifying instruments that require repair or replacement.
• When tracing sterilization practices at your organization, periodically open and inspect instruments in peel pouches and trays to ensure that they are appropriate for sterilization and -use.
• Create a “good catch” reporting process that rewards identification of instruments that should not be reprocessed so that tracking and trending is accurate for budgeting and quality purposes.
Patients face risks related to healthcare every day – do not let use of a soiled, damaged, or inappropriately reused SUD put patients or your health care organization at risk.
Sylvia Garcia-Houchins, RN, MBA, CIC, is director of infection prevention and control at The Joint Commission.
References:
1. McDermott KW, Liang, L. Agency for Healthcare Quality and Research, Healthcare Cost and Utilization Project, Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2018. STATISTICAL BRIEF #281 August 2021 Accessed at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb281-Operating-Room-Procedures-During-Hospitalization-2018.jsp
2. Research and Markets. United States Outpatient Surgical Procedures Market 2019-2023: Rising Number of Outpatient Surgical Procedures in ASCs, HOPDs, and Physicians' Office. Jan. 24, 2019 PRNewswire Accessed at: https://www.prnewswire.com/news-releases/united-states-outpatient-surgical-procedures-market-2019-2023-rising-number-of-outpatient-surgical-procedures-in-ascs-hopds-and-physicians-office-300783771.html
3. ECRI If It’s Not Clean, It’s Not Sterile: Reprocessing Contaminated Instruments. Event Reporting & Analysis – Alerts. Published 4/11/2017. Accessed at: https://www.ecri.org/components/PSOCore/Pages/e-lert041117.aspx
The Imperative of a Physician Partner for Every Infection Prevention Program
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the November 2021 issue of Healthcare Hygiene magazine.
There are currently tremendous improvement opportunities in the United States relative to the prevention of healthcare-associated infections (HAI), and the resulting patient harm and organizational cost. The Centers for Disease Control (CDC) estimates that HAIs account for an estimated 1.7 million infections and 99,000 associated deaths each year.1 Prevention of HAI can result in savings of between $1,000 and $40,000 per patient depending on the specific infection and healthcare setting.2 Many variables affect the success of an IP program including the strong partnership between the infection prevention department and an infectious diseases (ID) physician.3,4
In many U.S. hospitals, an ID physician serves as the chairperson or co-chairperson of the infection prevention committee and may also assist the IP department as an advisor and partner. Where it exists, this role is often not compensated.5,6 “Administrative time” is sometimes granted, but this time may or may not be spent in activities supporting the infection prevention program. The same ID physician is typically responsible for the antibiotic stewardship program (ASP) as well as clinical patient care including ID consults, which are each separate and apart from the infection prevention program.
In a 2015 study assessing physician resourcing for ASP and infection prevention, the researchers reported an average staffing level for the combined programs of 1.21/100 beds.7 Five years later, in a study by Stone still in press, only 49 percent of the hospitals reported the presence of a physician partner for the IP program, and most (71 percent) were only part-time.8
The Society for Healthcare Epidemiology of America (SHEA) whitepaper from 2015 states, “To effectively serve in these various roles, the physician partner for infection prevention programs requires formal support to protect time and effort for training activities and professional development.”9 However, in the absence of a regulatory mandate this does not happen universally. There is currently no requirement by the Centers for Medicare and Medicaid Services (CMS) or The Joint Commission (TJC) for a physician partner for infection prevention programs, at the local and/or corporate level.
Unfortunately, even when there is an ID physician designated to assist the infection prevention program, there is no guarantee that the physician has had the appropriate training regarding prevention and control of HAI, including outbreak investigation. There is currently no regulatory requirement for training of these physicians, and no process for certification. The Infectious Diseases Society of America (IDSA) offers an online course, though references the Association for Professional in Infection Control and Epidemiology (APIC) guidelines for specifics.10 SHEA also offers an online course, though covers only the basics.11 Neither provides certification.
Similarly, to date there is no standard role description for the physician partner for infection prevention programs in the U.S. However, there have been commentary and guidelines published. For example, the 2015 SHEA whitepaper proposes that the competencies required for this position should include:
• Data management and surveillance
• Leadership
• Microbiology and laboratory diagnostics
• Outbreak investigation
• Prevention and control of healthcare associated infections
• Quality improvement science
• Training and teaching methods and principles9
These competencies translate to a myriad of responsibilities which vary greatly from hospital to hospital, even within the same integrated delivery network (IDN). In addition to the core responsibilities of the ID physician (i.e., clinical patient care, antibiotic stewardship, ID consults), functions supporting the infection prevention department commonly include one or more of the following:
• Meet regularly with the infection prevention team to review ongoing issues/opportunity areas/work plan status
• Co-chair the Infection Prevention and Control Committee
• Co-Lead annual infection prevention work plan development based on results of risk analysis
• Partner with the infection prevention team on key performance improvement projects
• Interface with physicians to ensure compliance with products and practices supporting the infection prevention program (e.g., hand hygiene, isolation and personal protective equipment (PPE) use, disinfection and sterilization, line insertion, surgical prep)
• Provide physician training relative to infection prevention and control and outbreak investigation/mitigation
• Provide HAI case validation as needed by infection preventionists
• Partner with infection prevention program director to provide regular status reports to the local executive team (e.g., executive committee)
• In partnership with the infection prevention director, and execute authority as needed in the interest of patient safety (e.g., close the operating room or other units in the event of outbreak of infection, exposure, etc.)
A federal mandate for a dedicated ID physician for every local and corporate level infection prevention program would help to ensure that patient safety is supported with this critical role. This would in turn support the goal of zero preventable HAIs, with the associated reduction of patient suffering and healthcare costs. To best support reliable design, the mandate would ideally also require standard training and certification, development of a standard role description, and dedicated resourcing. It would seem reasonable to think that APIC, SHEA and IDSA could collaborate to support this.
References:
1. Patient Care Link: https://patientcarelink.org/improving-patient-care/healthcare-acquired-infections-hais/
2. Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013 Dec 9-23;173(22):2039-46. doi: 10.1001/jamainternmed.2013.9763.
3. McQuillen DP, Petrak RM, Wasserman RB, Nahass RG, Scull JA, Martinelli LP. The value of infectious diseases specialists: non-patient care activities. Clin Infect Dis. 2008 Oct 15;47(8):1051-63. doi: 10.1086/592067.
4. Barnes S, Spencer M. Reliable design of IP programs, Infection Control Today. Sept 2015. pp 41-43.
5. Zahn M, Adalja AA, Auwaerter PG, et al. Infectious Diseases Physicians: Improving and Protecting the Public's Health: Why Equitable Compensation Is Critical. Clin Infect Dis. 2019;69(2):352-356. doi:10.1093/cid/ciy888
6. Wright S. et al. Expanding Roles of Healthcare Epidemiology and Infection Control in Spite of Limited Resources and Compensation. Infection Control and Hospital Epidemiology. February 2010, vol. 31, no. 2.
7. Dickstein Y, Nir-Paz R, Pulcini C, Cookson B, Beović B, Tacconelli E, Nathwani D, Vatcheva-Dobrevska R, Rodríguez-Baño J, Hell M, Saenz H, Leibovici L, Paul M. Staffing for infectious diseases, clinical microbiology and infection control in hospitals in 2015: results of an ESCMID member survey. Clin Microbiol Infect. 2016 Sep;22(9):812.e9-812.e17. doi: 10.1016/j.cmi.2016.06.014. Epub 2016 Jun 29.
8. Stone PW, Dick A, Pogorzelska M, Horan TC, Furuya EY, Larson E. Staffing and structure of infection prevention and control programs. Am J Infect Control. 2009 Jun;37(5):351-357. doi: 10.1016/j.ajic.2008.11.001.
9. Kaye K et al. Guidance for Infection Prevention and Healthcare Epidemiology Programs: Healthcare Epidemiologist Skills and Competencies. Infect Control Hospital Epidemiol. Volume 36, Issue 04. April 2015, pp 369 – 380.
10. IDSA online course https://www.idsociety.org/clinical-practice/infection_prevention_and_control/infection-prevention-and-biopreparedness/
11. SHEA online course: https://www.shea-online.org/index.php/education/10-education/62-online-education
Safe Injection Practices in Ambulatory Care Settings
By Margaret M. Miller, BS, MT (ASCP) M, CIC, FAPIC, and Christina Michalek, BS, RPh, FASHP
This column originally appeared in the October 2021 issue of Healthcare Hygiene magazine.
Injection safety is a set of practices followed to perform injections in a manner that is safe for patients, staff, and others. Injection safety also includes practices to prevent sharps injuries and contamination of medications.
The primary objective in injection safety is preventing transmission of hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV). In response to the HIV/AIDS epidemic in the early-mid 1980s, Universal Precautions were created by the Centers for Disease Control and Prevention (CDC) and applied blood and body fluid precautions to all patients, regardless of infection status. Universal Precautions guidelines recommended the use of personal protective equipment to protect mucous membranes, hand hygiene, and methods for safely handling needles and other sharp devices; this concept became integral to the Occupational Safety and Health Administration (OSHA)’s 1991 regulation on occupational exposure to bloodborne pathogens in healthcare settings. In 1996, the Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for Isolation Precautions in Hospitals replaced Universal Precautions with Standard Precautions, which we continue to follow to this day.
OSHA's Bloodborne Pathogens standard (29 CFR 1910.1030) revision in 2001 specified in greater detail the engineering controls, such as non-needle devices and other safety features, which must be used to reduce or eliminate worker exposure. The standard places these requirements on employers whose workers can reasonably anticipate contact with blood or other potentially infectious materials.
Despite recommendations from HICPAC and OSHA, outbreaks and patient exposures to potentially infectious bodily fluids continue to be reported. The largest outbreak of HCV occurred at an outpatient endoscopy center where a cluster of patients developed acute HCV infection after their procedures. An investigation uncovered unsafe injection practices including reuse of patient syringes to access propofol vials. The single-dose vials (SDVs) were then reused for subsequent patients, transmitting potentially contaminated medication to those patients. More than 50,000 patients were notified of exposure and the need for HBV, HCV, and HIV testing. The final cost of the outbreak was estimated to be $16 million to $21 million. In the end, every exposure was preventable and would not have occurred if staff had adhered to established safe injection practices.
Safe Injection Practices
Following safe injection practices and aseptic technique during the preparation and administration of medications is a priority. Always perform hand hygiene and work on a clean surface, preferably in a separate medication preparation area, not in a patient-care area. Other essential elements include:
• Never administer medications from the same syringe to more than one patient, even if the needle is changed. Needles and syringes are sterile, single-use items
• Never enter a vial with a used syringe or needle
• Do not use bags of intravenous (IV) solution as a common source of supply for more than one patient
• Limit the use of multi-dose vials (MDVs) and dedicate them to a single patient whenever possible
• Always use facemasks when injecting material or inserting a catheter into the epidural or subdural space
• Prepare injectable medications as close as possible to the time of administration, rather than in advance
Procedures identified as prone to unsafe injection practices include:
• Administration of sedatives and anesthetics for surgical, diagnostic, and pain management
• Administration of IV medications for chemotherapy, cosmetic procedures, and alternative medicine therapies
• Use of saline solutions to flush IV lines and catheters
• Administration of joint injections
Medication Safety Recommendations for Ambulatory Care Settings
Employ safe practices for IV push medications:
• Do not further dilute IV push doses of medication unless recommended by the drug’s manufacturer or supported by evidence in peer-reviewed biomedical literature
• Label all clinician-prepared syringes of IV push medications
• Never pre-label empty syringes in anticipation of use
• To the maximum extent possible, purchase commercially prepared, premixed parenteral products
Take steps to safeguard controlled substances:
• Stock the smallest sized container necessary to provide the typical ordered dose of a controlled substance to minimize the need for drug waste and limit the potential for diversion
• Proactively monitor controlled substance usage, waste procedures, and documentation
• Place sharps/pharmaceutical waste containers in areas where they can be consistently observed. Select containers with small openings that do not allow medication devices or waste to be shaken out, and where possible, secure containers so they cannot be easily removed
Avoid patient harm when using single- and multiple-dose vial containers:
• SDVs should be used on a single patient and should be discarded after use
• Ideally, even MDVs should be used for only 1 patient. If used for multiple doses, discard the MDV when the beyond-use-date, or the expiration date, is reached and any time the sterility of the vial is in question
Support a positive safety culture:
• Encourage staff to identify and report near miss/close call events as well as actual errors
• Share lessons learned about underlying weaknesses in processes, systems, or equipment that explain why an error happens
• Train, monitor, and coach staff on best practices
Sharps Safety
In addition to injection and medication safety, there are additional practices that can protect the worker from occupational injuries. Contaminated sharps should not be bent, recapped or removed. Sharps should be disposed of as soon as possible after use. Disposal receptacles should be puncture resistant, labeled as biohazardous, leak-proof, and closable. Containers should be located as close as possible to the area of use and be large enough to fit a contaminated sharp without bending. Additionally, these receptacles should not be allowed to overfill, as doing so prevents the lid from closing properly.
Employers should maintain a confidential sharps injury log to document percutaneous injuries from all sharps. The event reporting system should document the type and brand of device involved in the incident, the work area where the exposure incident occurred, and an explanation of how the incident occurred.
ECRI is affiliated with the Institute for Safe Medication Practices (ISMP), a global leader in medication safety. For more than 25 years, ISMP’s research and advocacy have improved clinical practices and policies to prevent critical medication safety errors and adverse events. ECRI is available to assist facilities. Visit www.ecri.org.
Resources:
• Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care
• Outbreak of Hepatitis C at Outpatient Surgical Centers. Public Health Investigation Report. December 2009. Southern Nevada Health District Outbreak Investigation Team, Las Vegas, Nevada. Available at: http://southernnevadahealthdistrict.org/download/outbreaks/final-hepc-investigation-report.pdf.
• Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings (2007)
• CDC’s Injection Safety
• CDC’s One & Only Campaign
• Institute for Safe Medication Practices
• OSHA Bloodborne Pathogens and Needlestick Protection
Margaret M. Miller, BS, MT (ASCP) M, CIC, FAPIC, is an infection preventionist with ECRI.
Christina Michalek, BS, RPh, FASHP, is a medication safety specialist at the Institute for Safe Medication Practices (ISMP).
Reprocessing Ultrasound Transducers
By Diane Cullen, RN, MSN, MBA, CIC
Editor's note: This column originally appeared in the September issue of Healthcare Hygiene magazine.
The Joint Commission receives many inquiries from healthcare organizations asking how to reprocess medical devices, and more specifically, about the requirements for reprocessing ultrasound transducers. Examples of questions received include: What level of reprocessing is required? What do I do if the manufacturer’s instructions indicate a level of reprocessing that does not match my use of the transducer? What ultrasound transducer reprocessing practices are surveyors looking for?
The Joint Commission has recently published an article which addresses reprocessing practices for ultrasound transducers (published in the July 2021 issue of Joint Commission Perspectives.) The article describes some common survey observations regarding ultrasound transducers as well as related standard and element for performance (EP) scoring locations. To develop procedures for reprocessing transducers and to remain compliant with The Joint Commission Standards, organizations are encouraged to follow The Infection Prevention Hierarchy. The Hierarchy is a systematic approach which, when followed, will ensure an organization has considered local, state, and federal regulations (which may require organizations to follow specific evidence-based guidance), Centers for Medicare and Medicaid Services (CMS) Conditions of Participation, and abides by (or resolves conflicts with) the manufacturer's instructions for use (IFU) for any specific product.
Because ultrasound transducers are medical devices, they are regulated by the Food and Drug Administration (FDA). The FDA has published a guidance document specific for diagnostic ultrasound devices which refers to another guidance document: Reprocessing Medical Devices in health Care Settings: Validation Methods and Labeling. This document uses the Spaulding Classification System to guide device manufacturers when developing reprocessing instructions for medical devices based on the intended use of the device. For example, ultrasound transducers (transducers) may be used on intact skin, mucous membranes or within sterile body cavities or tissues during procedures. Each of these intended uses would result in a different requirement for reprocessing, based on Spaulding criteria:
• If a transducer is used in a sterile body cavity or sterile tissues, it is considered a critical device and requires sterilization.
• If a transducer is used on non-intact skin or mucous membranes, such as a vaginal, rectal or oral, it is a semi-critical device and should undergo high-level disinfection.
• If a transducer is only used on intact skin, it is considered non-critical and, regardless of whether it is contaminated with blood, the minimum requirement is low level or intermediate level disinfection.
• If the transducer manufacturer reprocessing instructions indicate that an instrument should be high-level disinfected if used to assist with percutaneous procedures or if contaminated with blood, the organization must follow the manufacturer instructions for use unless they have evidence to negate that instruction from the manufacturer.
A transducer sheath (probe cover), considered a medical device by the FDA, must be approved for use as a barrier. Transducer sheaths cannot be interchanged with items that have not been approved for barrier use (e.g., transparent IV dressings) and are intended to be used during procedures to protect the transducer from body fluids. Sheaths are applied to surface transducers, endocavity transducers or transducer probes used in surgical procedures. There has been debate whether the use of a transducer sheath on any transducer would negate the need to perform the minimum level of disinfection specified in the reprocessing instructions, as required by the manufacturer. The FDA has stated that use of a transducer sheath does not affect the Spaulding Classification of the transducer (as these sheaths may leak or tear) and therefore would not change the minimum level of reprocessing required for the transducer based on clinical use, unless otherwise indicated by the manufacturer.
The Joint Commission expects all organizations to comply with instructions provided by the manufacturer based on intended use of the device. However, there are times that the device manufacturer’s instructions might not conform to rules about intended use (Spaulding Classification System) or may not provide enough information for the end user to determine the correct method for reprocessing. If these situations occur, then contact the manufacturer. If there is a question or possible conflict with the instructions provided, then it is the healthcare organizations responsibility to refrain from using the device until the manufacturer is contacted and differences are reconciled. It is also important to remember that if alternative instructions are provided to a staff member during conversation with the manufacturer, he or she should obtain those instructions in writing and present them at the time of survey.
In addition, if the manufacturer requires a specific product, The Joint Commission surveyor will look for that product at a healthcare organization or expect an organization to have contacted and resolved any issue related to compatibility of products used. If the manufacturer requires a certain time or temperature for reprocessing, The Joint Commission will expect a healthcare organization to establish a means and process to measure both.
Healthcare organizations could be cited for:
• Failing to produce the IFUs of medical equipment and devices reprocessed at the organization,
• Failing to follow the steps of cleaning and reprocessing described in the IFU (if no steps have been taken to resolve deviation)
• Failing to use the products indicated in IFU (unless compatibility of alternative products has been confirmed)
• Failing to provide adequate personal protective equipment to staff (per IFU and hazard assessment) to complete the reprocessing and
• Failing to provide training or assessment of competence for staff who perform those procedures.
The Joint Commission understands that instrument handling and processing can be complex and are available to help. We encourage healthcare staff to contact an infection preventionist at The Joint Commission with any questions at Ask SIG.
Diane Cullen, RN, MSN, MBA, CIC, is assistant director of the Standards Interpretation Group for The Joint Commission.
A Call for Standardized Automation: HAI Surveillance in the 21st Century
By Sue Barnes, RN, CIC, FAPIC
Editor's note: This column originally appeared in the August 2021 issue of Healthcare Hygiene magazine.
Prevention and control of healthcare-associated infections (HAIs) became a greater priority in U.S. hospitals after publication of the 1999 Institute of Medicine (IOM) report, To Err is Human, which focused on avoidance of preventable harm.1 One component of infection prevention and control (IP&C) programs is surveillance of HAIs, which was originally the primary function of the infection preventionist (IP). However, the scope of these programs has massively expanded ever since, with prevention and control of infections now the primary imperative, and surveillance just one tool. Infection surveillance data is used to measure the success of IPC interventions, to identify areas for improvement, to assess the benefit of innovative products and practices, and to meet public reporting mandates and pay for performance goals. In hospitals where semi-automated infection surveillance software is not available, HAI surveillance is accomplished manually. Both methods provide data which can be subject to inter-rater reliability deficiency, inaccuracy, and for the manual process subjectivity as well. 2,3
For manual surveillance these limitations are due in part to variable interpretation of the complex HAI definitions, but also to simple human error. In addition, though HAI definitions are standardized by the Centers for Disease Control and Prevention (CDC), the process for manual infection detection has never been standardized. Similarly, for infection surveillance software there is no standard infection detection algorithm, and this is variable among the many software programs. In addition, infection surveillance software report accuracy is dependent upon the completeness of clinical documentation in the electronic medical record (EMR).
Legislative Support and Funding for Infection Prevention and Control
The Health and Human Services department (HHS) recently announced an $80 million plan focused in part on information technology (IT) development, but for the public health sector, not hospitals.4 The current HHS National Action Plan to Prevent HAI addresses the limitations of hospital based HAI surveillance processes in general terms, though not cost of infection surveillance software or the lack of algorithm standardization for infection detection.5 The 2022 HHS Justification of Estimates for Appropriations Committees report reflects that there is $35.7 million designated to advance the generation of new knowledge and promote the application of proven methods for preventing HAIs.6 However, there is no mention of directing any of these funds toward standardizing algorithms for electronic HAI surveillance, or making these systems equally available to all hospitals.
Technology - Automated vs. Manual Surveillance and the Need for Standardization
The advent of automated infection detection by applying data mining algorithms to the EMR or via specialized add-on infection surveillance software has been shown to improve the quality of HAI data, reduce the time required when compared to manual infection surveillance, as well as assist in early outbreak identification.7-10 And given the data reporting burden of the CDC’s National Healthcare Safety Network (NHSN), the additional automation of data export to NHSN is reported to further protect IP&C resources.11 However, automation requires a level of information technology (IT) support not always accessible to IP&C departments, which in addition to the cost of these programs has resulted in slow adoption.12 In 2014 it was estimated that only 45 percent of U.S. hospitals used electronic infection surveillance systems.13 And many of the software programs available today do not completely eliminate the need for manual record review to confirm case finding. Kenrick Cato, a researcher at the Columbia University School of Nursing has proposed that, “If monitoring systems could be fully automated, it would revolutionize the way infection surveillance is done.”13 One study reports that this has be done in at least one location, further concluding that “automated detection of HAIs according to NHSN surveillance definitions, can bridge the gap between retrospective surveillance of HAIs and prospective clinical-decision-oriented HAI support.”14,15 According to a 2020 study, we are still at least a decade away from widely utilizing electronic HAI surveillance due in part to lack of focus and funding by government, which could make automated surveillance available to every hospital IP&C department.16
HAI surveillance remains central to prevention and control efforts; however, HAI case finding as well as reporting to NHSN is resource-intensive, subject to inaccuracy due human error, and is hampered by a lack of standardization of manual and automated infection detection processes, which also challenges accurate inter-facility comparison. In addition, HAI data is retrospect, which prevents intervention in real time to expedite effective treatment.
To ensure that HAI data serves to optimally support IPC programs and meet reporting requirement, improvements in surveillance systems must keep pace with the technology available. The path to this end point would be expedited with government dollars directed in support of fully automated and standardized HAI surveillance, made available to all US hospitals.
In the short term this will require hospital executives partnering with IPC departments to leverage the rapidly evolving field of infection detection software to ensure best use of resources.
Sue Barnes, RN, CIC, FAPIC, is an independent clinical consultant.
References:
1. Maaike S, van Mourik M, Perencevich EN, et al. Designing Surveillance of Healthcare-Associated Infections in the Era of Automation and Reporting Mandates. Clin Infectious Dis. Vol. 66, Issue 6. March 15, 2018, Pp. 970-976. https://doi.org/10.1093/cid/cix835
2. Nuttall J, et al. The inter-rater reliability of the diagnosis of surgical site infection in the context of a clinical trial. Bone Joint Res. 2016 Aug;5(8):347-52.
3. Lin MY, Hota B, Khan YM, et al. Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA. 2010 Nov 10;304(18):2035-41.
4. HHS. $80 Million in Funding https://www.hhs.gov/about/news/2021/06/17/hhs-announces-80-million-in-arp-funding-to-bolster-underrepresented-communities-in-public-health-it-workforce.html
5. National HAI Action Plan. https://health.gov/our-work/health-care-quality/health-care-associated-infections/national-hai-action-plan
6. AHRQ Mission Budget 2022. https://www.ahrq.gov/sites/default/files/wysiwyg/cpi/about/mission/budget/2022/FY2022_CJ.pdf
7. Branch-Elliman W, Strymish J, Gupta K. Development and validation of a simple and easy-to-employ electronic algorithm for identifying clinical methicillin-resistant Staphylococcus aureus infection. Infect Control Hosp Epidemiol. 2014 Jun;35(6):692-8.
8. DeLisle S, Kim B, Deepak J, et al. Using the electronic medical record to identify community-acquired pneumonia: toward a replicable automated strategy. PLoS One. 2013 Aug 13;8(8):e70944.
9. Pramono RX, Imtiaz SA, Rodriguez-Villegas E. A Cough-Based Algorithm for Automatic Diagnosis of Pertussis. PLoS One. 2016 Sep 1;11(9):e0162128.
10. Salmon M, Schumacher D, Burmann H, et al. A system for automated outbreak detection of communicable diseases in Germany. Euro Surveill. 2016;21(13).
11. Vostok J, Lapsley W, McElroy N, et al. Assessment of the burden of mandatory reporting of health care-associated infection using the National Healthcare Safety Network in Massachusetts. Am J Infect Control. 2013 May;41(5):466-8. doi: 10.1016/j.ajic.2012.05.021
12. Hebden JN. Slow adoption of automated infection prevention surveillance: are human factors contributing? Am J Infect Control. 2015 Jun;43(6):559-62.
13. Levingston S. Software That Helps Spot Sneaky Infections. Bloomberg Terminal online. Dec. 18, 2014. https://www.bloomberg.com/news/articles/2014-12-18/software-that-fights-infections-in-humans-at-the-hospital
14. Koller W, de Bruin JS, Rappelsberger A, et al. Advances In Infection Surveillance and Clinical Decision Support With Fuzzy Sets and Fuzzy Logic. Stud Health Technol Inform. 2015;216:295-9.
15. Classen D, Li M, Miller S, et al. An Electronic Health Record–Based Real-Time Analytics Program for Patient Safety Surveillance and Improvement. Health Affairs. Vol 37., No 11. November 2018. https://doi.org/10.1377/hlthaff.2018.0728
16. Streefkerk H, Roel A, Verkooijen Roel PAJ, et al. Electronically assisted surveillance systems of healthcare-associated infections: a systematic review. Euro Surveill. 2020;25(2):pii=1900321. https://doi.org/10.2807/1560-7917.ES.2020.25.2.1900321
What You Need to Know when Considering Supplemental Methods for Terminal Room Disinfection: Ultraviolet-C Light Versus Hydrogen Peroxide Vapor
By Margaret M. Miller, BS MT (ASCP) M CIC FAPIC
Editor's note: This column originally appeared in the July 2021 issue of Healthcare Hygiene magazine.
For tables, see the July 2021 issue at: https://www.healthcarehygienemagazine.com/monthly-issues/
Many facilities seek to reduce healthcare-associated infections (HAIs) and transmission of viruses, multi-drug resistant organisms (MDROs), Clostridioides difficile (C. difficile) and other pathogens. The COVID-19 pandemic has highlighted the importance of environmental cleaning. To properly disinfect, one must manually clean visibly dirty surfaces prior to disinfection, use an EPA-approved disinfectant, and follow the application and dwell time instructions on product labels. There may be practice gaps in environmental cleaning practices, knowledge gaps in disinfection methods, and product variability due to supply chain challenges.
Supplemental no-touch disinfection methods provide an additional level of environmental decontamination following routine surface cleaning and disinfection. Here we will discuss considerations for implementing two of these technologies: Ultraviolet-C light (UV-C) and hydrogen peroxide vapor (HPV). Studies have shown when used after manual cleaning, UV-C and HPV disinfection methods significantly reduce contamination on environmental surface pathogens such as MDROs and C. difficile.
Supplemental disinfection devices decrease reliance on operators and potentially improve the efficacy of environmental surface cleaning, and specifically, terminal room disinfection. These devices are intended to supplement good cleaning protocols already in place, not to replace routine cleaning.
Implementation and operational considerations
Workflows for UV-C and HPV methods are similar; however, HPV requires additional steps and precautions. Both supplemental methods require the room to be unoccupied, manual cleaning and disinfection performed, room preparation, and cycle time. Key criteria for UV-C room disinfection are dose delivery, motion sensors with auto shut-off for safety, maneuverability, and lamp damage protection. HPV devices may be mobile or permanently installed in a space. HPV requires heating, ventilation, air conditioning vents and smoke detectors be blocked. Doors to the space must be sealed with tape to prevent HPV leakage. After the HPV cycle, a hydrogen peroxide sensor is used to verify the chemical concentration is less than one part per million (the exposure limit permitted by the Occupational Safety and Health Administration) prior to room entry.
Relative advantages and disadvantages of UV-C and HPV disinfection
Regardless of the type of supplemental disinfection device, thorough cleaning is required before the device is used because inorganic and organic materials that remain on surfaces interfere with the effectiveness of the process.
For UV-C technology, advantages include ease of use, the documented studies showing pathogen reduction in the environment after outbreaks and with high-risk pathogens in high-risk units, and the relatively quicker cycle times compared to HPV technology. As considerations for disadvantages, UV-C technology requires time, energy and expenses to implement effectively. Use increases room downtime between patients, compared to no supplemental disinfection, and its effectiveness may vary based on positioning in the area and system settings. Lastly, UVC disinfection is limited to solid surfaces, and some pathogens (Candida auris, C. difficile) may require longer exposure times.
For HPV technology, the system’s effectiveness is not affected by physical constraints or shadows and thus can effectively disinfect an enclosed space within one cycle. Unlike UV-C disinfection, HPV can be used with both compatible porous and non-porous surfaces. For disadvantages compared to UV-C disinfection, use of HPV increases room downtime and may last several hours depending on size and the need for the operator to seal all HVAC vents, smoke detectors, and outer doors. Lastly, HPV may damage medical and nonmedical equipment if hydrogen peroxide is not approved for use in manufacturer’s instructions for cleaning. Items not compatible must be appropriately covered or removed from the room during disinfection.
In summary, the COVID-19 pandemic has increased interest in supplemental disinfection devices for use in various healthcare settings. ECRI member interest more than doubled in 2020. Organizations may buy and implement these systems without full knowledge of the requirements for safe and effective system use. Misuse of devices can result in human chemical exposure and potential damage to sensitive devices, equipment, and items. It is important to select the type of system that meets your needs, develop an implementation plan, and identify high-risk areas for use to strategize to reduce bioburden and healthcare related infections in your facility.
UV disinfection systems are not classified as medical devices and therefore are not regulated by the Food and Drug Administration. Furthermore, there are no standardized test methods for comparing systems so your team may need help. Facilities should regard manufacturer claims critically; be sure to read the fine print, and look for citations that provide study details. ECRI is available to assist facilities through the process of device selection, implementation and assessing the effectiveness of a device in your space. Visit www.ecri.org and follow @ECRI_Org on Twitter.
Margaret M. Miller, BS, MT (ASCP)M, CIC, FAPIC, is an infection preventionist with ECRI.
References:
CDC. Disinfection and Sterilization. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ . Accessed June 25, 2021.
US Agency for Healthcare Research and Quality. No-Touch Modalities for Disinfecting Patient Rooms in Acute Care Settings: A Rapid Review. Oct 2020.
The Lancet. D Anderson et al. Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR Disinfection). August 2019.
ECRI. Dry Hydrogen Peroxide Disinfection Systems for Reducing Healthcare-associated Infections. November 2020.
ECRI. Evaluation Background: UV Room Disinfection Devices. Updated April 2020.
Department of Industrial Relations. Permissible Exposure Limits for Chemical Contaminants. Accessed June 25, 2021.
ECRI. Ultraviolet Light Air-purification Systems for Preventing Healthcare-associated Infections. March 2020.
ECRI. Using UV Disinfection Safely and Effectively: Technology Challenges during the COVID-19 Pandemic. May 2020.
American Journal of Infection Control (AJIC). D Weber et al. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. May 2016.
ECRI. Use of Automatic Chemical Disinfection Systems May Lead to Chemical Exposure and Equipment Damage. July 2020.
ECRI. UV Room Disinfection: Estimating Labor Costs and Understanding Common Use Scenarios. October 2017.
Preparation for Survey: Tips for Infection Preventionists
By Sylvia Garcia Houchins, MBA, RN, CIC
Editor's note: This column originally appeared in the June 2021 issue of Healthcare Hygiene magazine.
It is difficult for an infection preventionist (IP) to anticipate what to expect during a healthcare organization’s survey. With more than 30 years of experience as an IP, I have personally participated in 10 full, unannounced Joint Commission surveys, a few for-cause Joint Commission surveys, three intense occupational health and safety visits, and several state surveys. So, how does one become “good at survey” when the opportunities may be few and far between?
During the first few surveys, I was allowed to accompany the physician, nurse, administrator, and life safety surveyor. All were terrific teachers, and I learned a lot from each. The experience also prepared me to act as a “guide” for surveyors during subsequent surveys and provided me the confidence to explain to surveyors why I did not think my organization was out of compliance with the Joint Commission standards or Centers for Medicare and Medicaid Services’ (CMS) Conditions of Participation (CoP).
IPs should be proactive in asking to be allowed to participate in surveys and should not wait to be asked. Instead, IPs should volunteer to participate in mock surveys – even if they are not infection control- related. The experience will help provide information on how an organization functions during survey, its processes that are in place, who is who on its team, as well as the survey process itself.
IPs should use a systematic approach to determining compliance with regulatory requirements. For example, before working at the Joint Commission, and while working as a consultant, I helped many organizations prepare for their surveys and respond to adverse survey results including immediate jeopardy. I always followed a hierarchical approach to compliance. First, I determined if there was a law or regulation that applied, followed by ensuring compliance with CMS CoPs that included researching and interpreting instructions for use and seeking clarity from manufacturers. Then, if needed, I always turned to locating supporting literature, evidence-based guidelines, and best practices to help.
In April 2019, this approach was published in the Joint Commission’s publications, Perspectives, to help organizations learn how become compliant with Joint Commission infection prevention and control standards and to provide a good starting point for preparing for a survey. But, merely following the approach is not enough. Most IPs are incredibly dedicated and resourceful, but I find that many overthink or underthink what is required for survey. Below are some “tips” that will help an IP prepare for survey.
1. Make sure your organization can provide evidence that the IP is qualified and competent. There is an important distinction between qualified and competent and we often find that organizational leadership does not understand the difference. The following information provides clarity on the differences as well as an example of what could be identified as an issue during survey.
Qualifications: Healthcare organizations must define an IP’s qualifications based on his or her specific-to-job responsibilities. Qualifications for infection control may be met through ongoing education, training, experience and/or certification. For example, organizations may define an IP’s qualifications as “ongoing experience practicing in the infection prevention and control field as well as initial and ongoing infection prevention-specific education and training.” Or, they may require that, “The IP has three years of experience in a surgical setting and certification in infection prevention and control or able to obtain certification within two years of hire.”
Competency: This (see Joint Commission standard HR.01.06.01) differs from education and training in that competency incorporates knowledge, technical skills, and ability. All are required to deliver safe care correctly and perform technical tasks. Assessing competency, then, is the process by which the organization validates, via a defined process, that an individual has the ability to perform a task consistent with the education and training provided. An example of a competency assessment could be the validation that an IP can perform surveillance correctly by providing cases studies and determining that the IP correctly identifies whether the case meets the definition of a healthcare-associated infection. Competency may also include, confirming that he or she correctly identifies the type of infection, correctly calculates infection rates, and presents results in a clear manner with relevant interpretation.
A common mistake that is scored during survey happens when an IP who is not competent themselves, signs off on the competency of a person who is performing a task, for example high-level disinfection or sterilization. The IP may be knowledgeable or have training related to disinfection and sterilization, but he or she is not competent to perform the tasks and identify and problem solve when issues arise. In the example, of sterilization, the IP knows the basic steps for reprocessing instruments but could not assess that each step is being done correctly or how to identify that an instrument or piece of equipment should be taken out of service.
2. It is necessary for an IP to thoroughly understand his or her primary resources. Some of the most important resources include:
• Core Infection Prevention and Control Practices for Safe Healthcare Delivery in All Settings – Recommendations of the Healthcare Infection Control Practices Advisory Committee, 2017. Section five of this document explains requirements of standard precautions but more importantly it provides a framework for infection prevention and control in any health care setting.
• OSHA Bloodborne Pathogens Standard and Personal Protective Industry Standard. These standards are required by law. All accredited organizations must comply with state and federal laws and regulations.
• Centers for Disease Prevention and Control (CDC) Guidelines and Guidance Library. This is the best place to search for FREE evidence-based guidance.
• Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the HICPAC. This is an important webpage for any organization that reprocesses flexible endoscopes.
• Manufacturer instructions for use for any medical device, supply or equipment that needs to be or is used to clean, disinfect, or sterilize.
These resources provide basic information to guide development of any organization’s infection control plan.
3. IPs should perform tracers like a Joint Commission surveyor does. Surveyors don’t follow a checklist; they ask open-ended questions and determine through the answers how to identify risks. For example, an IP might perform a “procedure” tracer. In doing so, he or she selects a device in sterile processing that is used by a clinic. Then the IP asks the clinic nurse or medical assistant what procedure(s) are conducted in the clinic with that device and with what additional equipment or devices does he or she prepare for the procedure? This provides context for how the devices are used.
IPs should watch how the health care organizations staff prepares a room for the next patient. They should also ask for instructions for use and if they are easily available. Then, review them to determine if items were used, discarded, or reprocessed in accordance with the instructions. Make sure that the reprocessing level and intended use match for everything used. If an item is cleaned, disinfected, or sterilized, follow it through the process, ask open ended questions without assuming know the answer. Don’t make assumptions. Ask clarifying questions, like: Does it make sense? IPs should make sure that they thoroughly understand the processes involved and identify any risk points. Then they can use the information to identify other “threads to pull” prioritize and mitigate risks.
4. Access resources that The Joint Commission offers through www.jointcommission.org Scroll to the bottom of the page to find a link to the Joint Commission Connect website. Every Joint Commission-accredited health care organization has access. If your organization cannot access, ask your accreditation manager to give you access. Through this website you can access:
• E-dition is the electronic version of the Joint Commission standards and all IPs should access to determine the standards that apply to their organization.
• Perspectives is the official publication of the Joint Commission. If an infection control process or scoring example is addressed in this publication it is important that the IP or accreditation team evaluate organizational compliance. A recent example is Blood Glucose Monitoring and Insulin Administration which was published in the Consistent Interpretations section. The CDC has issued multiple alerts that unsafe practices during assisted monitoring of blood glucose and insulin administration place people at risk of transmission of bloodborne viruses (HBV, hepatitis C virus, and HIV) and have since 2008, linked these unsafe practices to outbreaks of viral hepatitis related to healthcare, including Personal Care Homes, Assisted Living, Home Care Agency, Long-term Care. Review of scoring has identified knowledge gaps amongst providers and\or leaders that have resulted in unsafe practices and subsequent escalation to an Immediate Threat to Health and Safety. For deemed organizations, CMS requires that state and accreditation organizations refer any infection control breaches that could potentially expose patients to the blood or bodily fluids of another to the appropriate state public health authority (S&C: 14-36-ALL REVISED 10.28.16). In July 2021, the Joint Commission will publish clarification on how compliance with reprocessing of ultrasound transducers will be scored. Prior topics include water management, personal protective equipment, surgical attire, and food and drink in patient care areas. IPs should review scoring examples to identify if interventions are needed to ensure patient safety.
• Ask a Standards question link. By accessing this link, IPs can ask a certified infection preventionist a question and have it answered. It will not result in notification of a surveyor or have any impact on the survey (other than helping clarify a requirement). IPs can ask for an email or a phone response. For complex questions, ask to schedule a phone call. If you feel that you need the answer in writing, ask if you can summarize the key points that you heard and send it to the to confirm or clarify the discussion.
Most IPs are responsible for ensuring organizational compliance to Joint Commission infection prevention and control standards. By following the aforementioned “tips” they can help ensure their healthcare organization is compliant with Joint Commission standards. The Joint Commission would love to hear from IPs. Submit suggestions for additional useful information, send feedback or ask a question though The Joint Commission’s “Ask a Standards Question” link.
Sylvia Garcia Houchins, MBA, RN, CIC, is the infection control and prevention director for the Joint Commission
Using a Post-Operative Surgical Site Infection Prevention Bundle to Reduce Risk
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the May 2021 issue of Healthcare Hygiene magazine.
Guidelines addressing the prevention of surgical site infections (SSI), using a bundle of measures, include those from the Centers for Disease Control and Prevention,1 the American College of Surgeons,2 the World Health Organization3 as well as a nationally accepted protocol called Enhanced Recovery After Surgery (ERAS).4 Each of the SSI prevention guidelines focus on the pre-operative and intra-operative periods, with little or no information regarding the post-operative period.5 And while the ERAS protocol(s) does address the post-operative period, it does not address infection risk in that period. The surgical incision(s) does not begin to heal until 48 hours days post-op when epithelialization of the wound occurs, and until then it is possible for contamination to occur which can lead to infection.6
Wound Closure and SSI Risk
Wound closure is applied during the intraoperative period, but remains in place, and impacts incisional healing and the risk of post-operative SSI. There is wide variation in clinical guidance regarding wound closure and conclusive direction is missing from the current SSI prevention guidelines. Consequently, wound closure is primarily guided by surgeon choice. Wound closure types include non-impregnated suture, antimicrobial impregnated suture, staples, steri-strips, surgical glue (2-octyl-cyanoacrylate) and sometimes a combination of glue over suture or steri-strips. There is evidence that the following wound closure types can increase surgical infections risk: staples,7,8 non-impregnated suture.9 Alternatively, there is evidence that the following wound closure types reduce surgical infection risk: antimicrobial (triclosan) impregnated suture9, surgical glue (2-octyl-cyanoacrylate), or a combination of glue over suture.8
Post-Operative Wound Dressing and SSI Risk
Gauze and tape dressings, the original post-op dressing, were designed to protect the incision from disruption. During the past decade there have been numerous innovations in post-op incisional dressings. Today, these innovative dressings are designed to not only protect the incision, but also promote an optimal healing environment, and reduce the risk for microbial growth. And yet, arguable the most common type of post-operative dressing remains gauze and tape, because as with other post-op procedures, clear direction is missing from the SSI prevention guidelines. Consequently, dressing choice is based mostly on surgeon preference. Innovations in post-op dressings for which there is evidence of reduced infection risk include those that are absorbent, transparent and CHG-impregnated,10 silver-impregnated,11,12 and negative pressure wound dressings.13
Post-Operative Bathing and SSI Risk
Directions given to patients after surgery, commonly include a restriction from bathing for 48 to 72 hours, presumably to prevent irritating or macerating the wound, and disturbing the healing environment. However, there is no science supporting this restriction, and reducing hygiene may result in accumulation of sweat and dirt on the body, increasing the risk of wound contamination. Since clear direction regarding post-op bathing is missing from the SSI prevention guidelines, this is commonly left to the surgeons’ discretion. According to one clinical paper which reviewed nine studies involving 2,150 patients, no increased incidence of infection was found in the patients allowed to shower or bathe as a part of their normal daily hygiene before suture removal compared with those who were instructed to keep the site dry until suture removal.14 Given that all SSI prevention guidelines recommend bathing with chlorhexidine (CHG) soap or impregnated CHG wash cloths before surgery, some hospitals are reasonably concluding that post op bathing with CHG for a few days until the incision begins to heal, is prudent.15
Nasal Colonization and SSI Risk
Nasal decolonization has long been reported in peer reviewed studies as an important tool when used in a bundle, to reduce the risk of post-operative surgical site infections. However, the studies have all focused only on preoperative application of nasal decolonizing agents. This may be because most of the studies were performed using mupirocin which requires five consecutive days of application, and then provides Staph aureus elimination for up to 87 percent after four weeks, and 48 percent after six months.16 Now that nasal antiseptics are taking the place of mupirocin due to the immediacy of effect, as well as the emergence of mupirocin resistant Staph aureus, some hospitals are continuing the application of the alcohol-based nasal antiseptic for a few days until incisional healing begins.17
Conclusions
While a bundle approach to SSI prevention has been recommended by the existing clinical SSI prevention guidelines, none address the post-operative period of incisional healing. There is evidence to recommend at least four prevention measures that reduce the risk of SSI during the post-operative period:
1. Wound closure with antimicrobial impregnated suture, surgical glue, or a combination of the two
2. An innovative post-operative dressing supported by peer reviewed clinical evidence of efficacy
3. Bathing/showering post-operative days 1-4, with 4 percent CHG soap or 2 percent CHG-impregnated bathing cloths
4. Twice daily nasal decolonization with alcohol based nasal antiseptic post-operative days 1-4.
References:
1. Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605-627. doi:10.1086/676022
2. Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017 Jan;224(1):59-74. doi: 10.1016/j.jamcollsurg.2016.10.029.
3. Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2018. PMID: 30689333.
4. Pędziwiatr M, Mavrikis J, Witowski J, et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. 2018;35(6):95. Published 2018 May 9. doi:10.1007/s12032-018-1153-0
5. Caruso TJ, Wang EY, Schwenk H, Marquez JLS, Cahn J, Loh L, Shaffer J, Chen K, Wood M, Sharek PJ. A Postoperative Care Bundle Reduces Surgical Site Infections in Pediatric Patients Undergoing Cardiac Surgeries. Jt Comm J Qual Patient Saf. 2019 Mar;45(3):156-163. doi: 10.1016/j.jcjq.2018.05.009.
6. Lawrence WT. Physiology of the acute wound. Clinics in Plastic Surgery 1998;25(3):321‐40.
7. Carli AV, Spiro S, Barlow BT, Haas SB. Using a non-invasive secure skin closure following total knee arthroplasty leads to fewer wound complications and no patient home care visits compared to surgical staples. Knee. 2017 Oct;24(5):1221-1226. doi: 10.1016/j.knee.2017.07.007.
8. Ando M, Tamaki T, Yoshida M, Sasaki S, Toge Y, Matsumoto T, Maio K, Sakata R, Fukui D, Kanno S, Nakagawa Y, Yamada H. Surgical site infection in spinal surgery: a comparative study between 2-octyl-cyanoacrylate and staples for wound closure. Eur Spine J. 2014 Apr;23(4):854-62. doi: 10.1007/s00586-014-3202-5.
9. Renko M, Paalanne N, Tapiainen T, Hinkkainen M, et al. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: A double-blind, randomised controlled trial. Lancet Infect Dis. 2016 Sep 19. pii: S1473-3099(16)30373-5. doi: 10.1016/S1473- 3099(16)30373-5
10. Bashir, MH, Olson LK and Walters SA. (2012). Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J Infect Control, 40(4), 344-348. doi:10.1016/j.ajic.2011.03.030
11. Abboud, E. C., Settle, J. C., Legare, T. B., Marcet, J. E., Barillo, D. J., & Sanchez, J. E. (2014). Silver-based dressings for the reduction of surgical site infection: review of current experience and recommendation for future studies. Burns, 40 Suppl 1, S30-S39. doi:10.1016/j.burns.2014.09.011 25.
12. Connery SA, Downes KL and Young C. (2012). A retrospective study evaluating silver-impregnated dressings on cesarean wound healing. Adv Skin Wound Care, 25(9), 414-419.
13. Norman G, Goh EL, Dumville JC, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020;6(6):CD009261. Published 2020 Jun 15. doi:10.1002/14651858.CD009261.pub6
14. Dayton P, Feilmeier M, Sedberry S. Does postoperative showering or bathing of a surgical site increase the incidence of infection? A systematic review of the literature. J Foot Ankle Surg. 2013 Sep-Oct;52(5):612-4. doi: 10.1053/j.jfas.2013.02.016.
15. Children’s Mercy Post Op Bundle: https://www.childrensmercy.org/siteassets/media-documents-for-depts-section/documents-for-health-care-providers/evidence-based-practice/critically-appraised-topics/ssi-postoperative-surgery-bundle.pdf accessed April 21, 2021
16. Doebbeling BN, Breneman DL, Neu HC, Aly R, Yangco BG, Holley HP Jr, Marsh RJ, Pfaller MA, McGowan JE Jr, Scully BE. Elimination of Staphylococcus aureus nasal carriage in health care workers: analysis of six clinical trials with calcium mupirocin ointment. The Mupirocin Collaborative Study Group. Clin Infect Dis. 1993 Sep; 17(3):466-74.
17. Franklin S. A safer, less costly SSI prevention protocol-Universal versus targeted preoperative decolonization. Am J Infect Control. 2020 Dec;48(12):1501-1503. doi: 10.1016/j.ajic.2020.02.012.
Developing a Respiratory Protection Program
By Hillary Hei, MPH, CIC, LSSGB
This column originally appeared in the April 2021 issue of Healthcare Hygiene magazine.
The response to the COVID-19 pandemic has emphasized the need for adequate protection against SARS-CoV-2 to ensure employee safety. Prior to the pandemic, most healthcare settings outside of acute care rarely needed to wear respiratory protection beyond a medical-grade facemask. With this abrupt shift in the need for respirator use, many facilities using respirators for the first time also need to adhere to and maintain compliance with federal regulations. If respirators are used in a workplace, the U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA) requires employers to follow the Respiratory Protection Standard 29 CFR 1910.134, or OSHA state-plan equivalent.1 This standard states that if staff must wear a respirator due to an identified hazard at work, a written respiratory protection program (RPP) is required.
From the start of the pandemic through Dec. 31, 2020, OSHA has issued numerous citations from more than 300 inspections for violations relating to COVID-19,2 identified either by complaints, referrals or severe incident reports. This has totaled more than $3.9 million in proposed penalties across many industries, with healthcare settings including hospitals, nursing homes, and long-term care settings.
Whether your facility is experienced with an RPP or not, it is best to ensure your program is in compliance with OSHA regulations and that the program can be sustainably maintained. OSHA’s Respiratory Protection standard 29 CFR 1910.134 has legally enforceable requirements when respirators are used, including:
- Designate a respirator program administrator (RPA).
Each facility must designate one individual to be like an air-traffic controller for the complete RPP. This person is in charge of setting up and overseeing the program, with specific tasks such as maintaining all necessary records and arranging annual trainings and fit tests. This person does not need to be a licensed healthcare professional but should be “qualified by appropriate training or experience1.”
- Develop a written workplace-specific RPP and update as necessary.
The facility’s RPP must be relevant and specific to the worksite. OSHA also emphasizes the need to keep the program updated as necessary. Anytime a policy or procedure related to respirator use changes, this must be reflected in the RPP. - Complete a hazard assessment.
A hazard assessment is essential to identify potential hazardous exposures that require the use of respiratory protection while employees are on the job. It is important to consider all employee roles, duties, and responsibilities while performing this assessment. - Select the respirators that will be used based on the hazard assessment.
In healthcare settings, potential exposures to infectious pathogens may warrant protection against droplet or airborne transmission. If respirators are needed to provide reduced exposure to airborne hazards, it’s important to specify in your RPP which type(s) of respirator the facility will use (e.g. N95s, elastomeric facemasks). - Conduct medical evaluations.
Because respirator use may exacerbate underlying medical conditions, medical evaluations are required for each employee prior to donning a respirator for work or fit testing. Information solicited from evaluation questionnaires must be reviewed by a physician or other licensed healthcare professional, and the employee must be approved prior to using a respirator. - Complete respirator fit testing.
Fit testing is the most important part of the respirator program, and OSHA requires fit testing for all users of tight-fitting respirators. The test ensures that the brand make and size adequately reduces employee exposure from airborne hazards. This fit test must be repeated annually, whenever the employee reports changes in their physical condition, or when a different brand, type, or size of respirator is introduced to the employee for use. Facilities can perform fit tests through either a qualitative or quantitative fit test. - Educate employees.
Training is also essential, as staff must understand the limitations and capabilities of the respirator used. Users should know when a respirator is necessary, how to inspect, put on, and properly take off a respirator, and how to properly perform a seal check.
- Maintain all necessary records.
The RPP requires all records to be maintained and readily available to staff and OSHA upon request. Staff medical questions, medical evaluation and clearance, fit testing records, and documentation of staff training must be kept.
The response to the pandemic has created an increased demand for respirators such as N95s and has resulted in supply chain issues in respirators and fit testing kits. OSHA is temporarily exercising enforcement discretion on a case-by-case basis when considering issuing citations related to improper respiratory protection during COVID-19. Facilities must demonstrate and document “good-faith” efforts to comply with OSHA standards and any interim enforcement memoranda.3 Interim provisions with altered enforcement include postponing annual staff fit testing,4 the use of respirators beyond the manufacturer’s recommended shelf life,5 extended use and limited reuse, the utilization of respirators from other countries not approved by NIOSH,6 and decontamination for reuse.7
Under the Biden administration, employers should expect increased enforcement by OSHA. Effective March 12, 2021, OSHA is now implementing a National Emphasis Program to ensure that employees in high-hazard industries are protected from SARS-CoV-2 transmission.8 The new directive emphasizes more resources to provide on-site workplace inspections, targeting healthcare settings such as hospitals, nursing homes and assisted living, and emergency response settings. In order to be prepared, review or create your worksite-specific RPP, update and edit old procedures, and ensure staff are educated in proper respirator use to ensure employee safety.
If you are in a long-term care setting and would like to learn more, visit ECRI (https://www.ecri.org/solutions/respiratory-protection-program-ltc) for a comprehensive learning module that provides an in-depth foundation on establishing and sustaining your own RPP.
Hillary Hei, MPH, CIC, LSSGB, is an infection preventionist with ECRI, an independent, nonprofit organization improving the safety, quality, and cost-effectiveness of care across all healthcare settings worldwide.
References:
- Standard 1910.134: Respiratory Protection. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134. Accessed March 22, 2021.
- OSHA National News Release. January 8, 2021. U.S. Department of Labor’s OSHA Announces $3,930,381 in Coronavirus Violations. https://www.osha.gov/news/newsreleases/national/01082021. Accessed March 22, 2021.
- OSHA Enforcement Memorandum. April 16, 2020. Discretion in Enforcement when Considering an Employer’s Good Faith Efforts During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.osha.gov/memos/2020-04-16/discretion-enforcement-when-considering-employers-good-faith-efforts-during. Accessed March 22, 2021.
- OSHA Enforcement Memorandum. March 14, 2020. Temporary Enforcement Guidance – Healthcare Respiratory Protection Annual Fit-Testing for N95 Filtering Facepieces During the COVID-19 Outbreak. https://www.osha.gov/memos/2020-03-14/temporary-enforcement-guidance-healthcare-respiratory-protection-annual-fit. Accessed March 22, 2021.
- OSHA Enforcement Memorandum. April 8, 2020. Expanded Temporary Enforcement Guidance on Respiratory Protection Fit-Testing for N95 Filtering Facepieces in All Industries During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.osha.gov/memos/2020-04-08/expanded-temporary-enforcement-guidance-respiratory-protection-fit-testing-n95. Accessed March 22, 2021.
- OSHA Enforcement Memorandum. April 3, 2020. Enforcement Guidance for Use of Respiratory Protection Equipment Certified under Standards of Other Countries or Jurisdictions During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.osha.gov/memos/2020-04-03/enforcement-guidance-use-respiratory-protection-equipment-certified-under. Accessed March 22, 2021.
- OSHA Enforcement Memorandum. April 24, 2020. Enforcement Guidance on Decontamination of Filtering Facepiece Respirators in Healthcare During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.osha.gov/memos/2020-04-24/enforcement-guidance-decontamination-filtering-facepiece-respirators-healthcare. Accessed March 22, 2021.
- OSHA Direction. March 12, 2021. National Emphasis Program – Coronavirus Diseases 20219 (COVID-19). https://www.osha.gov/sites/default/files/enforcement
Updating Standards to Improve Patient Safety: Water Management
By Sylvia Garcia-Houchins, MBA, RN, CIC
This column originally appeared in the March 2021 issue of Healthcare Hygiene magazine.
Even though the United States has one of the safest water systems in the world, the Centers for Disease Control and Prevention (CDC) has estimated that 7.2 million Americans get sick every year from diseases spread through water. Some of those illnesses occur while patients are receiving care within health care organizations. However, quantifying the actual number of those that occur within health care organizations is difficult. These illnesses may or may not be recognized as resulting from a water source and, even if recognized, still may not be reported to public health because the causative agent is not Legionella. Legionella is nationally reportable while other causative agents are not specifically required to be reported by certain State Health Departments. For example, Washington State has specific reporting requirements for waterborne outbreaks while other states have general requirements for reporting clusters or outbreaks that do not specify known or suspected waterborne disease.
Once reported, state and local health departments have jurisdiction over investigations within their state and may choose not to provide aggregated data on the results of those investigations. However, media reports periodically highlight outbreaks across the United States. When invited to assist with an outbreak investigation, the CDC Division of Healthcare Quality Promotion (DHQP) identified that 22 percent of consultations they conducted were water related. DHQP identified nontuberculous mycobacteria as the most frequently involved pathogen during their investigations (29.9 percent) but it identified an additional list of 20 organisms, including those that are frequently identified in clinical specimens such as Pseudomonas spp., Acinetobacter baumanii, and Enterobacter spp. The organization also found that the source of the outbreaks they investigated most often involved medical products (35.8 percent), and most of these products were medical devices (83.3 percent). DHQP findings emphasize the need to consider not only centralized water systems, but any devices that utilize water, as potential sources of waterborne disease. Patient waterborne infections identified by DHQP were determined to be preventable if the affected health care organization had utilized available information about prevention of waterborne pathogens and implemented an effective water management plan.
In 2017 (and updated in 2018), CMS published Requirement to Reduce Legionella Risk in Healthcare Facility Water Systems to Prevent Cases and Outbreaks of Legionnaires’ Disease. At that time, the Joint Commission did not make any changes to its standards as failure of hospitals, critical access hospitals, and nursing care centers to minimizes pathogenic biological agents in cooling towers, domestic hot- and cold-water systems, and other aerosolizing water systems was already being scored under standard EC.02.05.01 EP 14 (EP 6 for NCC).
Almost four years later, accredited organizations are still being cited out of compliance. And, more importantly, patients continue to develop preventable waterborne infections. There are a lot of reasons (e.g., more pressing priorities, lack of knowledge, misunderstanding of the requirements) for which organizations have not complied, developed, and implemented an effective water management program. As is the nation's oldest and largest standards-setting and accrediting body in healthcare, the Joint Commission has evaluated the situation and identified an opportunity ensure that organizations implement processes to reach zero harm from preventable waterborne disease. In its current form, EC.02.05.01 EP 14 (EP 6 in the Comprehensive Accreditation Manual for Nursing Care Centers) does not provide sufficient guidance and requirements to ensure organizations are implementing appropriate water management plans.
In October 2020, the Joint Commission published draft water management program standards for field review. Positive comments received verified some existing suspicions. The majority of responding healthcare organizations had a water management plan (90 percent) that addressed Legionella prevention but only 74 percent indicated that they addressed other waterborne pathogens. Based on feedback from field review and water experts at the CDC, the draft water management program standards were updated and approved by CMS, as required for deeming.
The new standard and EPs (EC.02.05.02, EPs 1 through 4) will be published July 1, 2021 in accreditation manuals for hospitals, critical access hospitals, and nursing care centers. This standard is effective January 1, 2022, giving organizations time to ensure that they have developed and implemented a fully compliant water management program. The new standard incorporates and clarifies expectations currently addressed during the survey process. It also includes additional steps and processes such as assigning responsibility and oversight for the water management program, and identifying water sources, treatment systems, and types of water containing equipment using a basic diagram. Prepublication access will be available in April.
Examples of possible situations relating to EC.02.05.01 EP 14 that would currently be scored as non-compliant were published in the January 2020 edition of Perspectives. These examples remain applicable even with implementation of the new standard – although scoring location could change.
Organizations should be aware of free, available CDC resources that can be used to assist them in identifying risks and prevention strategies, including general information, organism-specific information, and tools for investigating outbreaks.
The resources discuss:
• Creating a comprehensive water management program, including a water risk assessment and basic prevention measures to prevent waterborne disease including a water management checklist for healthcare facilities
• Guidance for identifying specific problem organisms
o Nontuberculous mycobacteria
o Pseudomonas aeruginosa
• Managing Outbreak
Most healthcare organizations have been aware of the need to identify potential sources and control infection from Legionella. Now it is time to look into risks related to other waterborne pathogens that may be “flying under the radar” and causing disease to facilities’ patients. By using the excellent resources that CDC has developed, proactively identifying potential sources within a healthcare organization, and implementing surveillance and control measures, waterborne pathogens can be prevented, detected and controlled.
Sylvia Garcia-Houchins is the infection control and prevention director at the Joint Commission.
Reference: 1. Perkins, K., Reddy, S., Fagan, R., Arduino, M., & Perz, J. (2019). Investigation of healthcare infection risks from water-related organisms: Summary of CDC consultations, 2014—2017. Infection Control & Hospital Epidemiology, 40(6), 621-626. doi:10.1017/ice.2019.60
Infection Prevention, Patient Safety and Quality – Which Came First?
By Sue Barnes, RN, CIC, FAPIC
Editor's note: This column originally appeared in the February 2021 issue of Healthcare Hygiene magazine.
The departments of patient safety, quality and infection prevention and control (IP&C) all support optimizing of healthcare outcomes and are driven by state and federal regulatory requirements. Safety departments aim to prevent or reduce patient injury and harm. Quality departments aim to increase the use of and adherence to evidence-based practices and to improve efficiency.1 IP&C is separate from, but supportive of, the objectives of both patient quality and patient safety by reducing the risk and rate of healthcare associated infections and controlling outbreaks of infectious disease.2 IP&C is a scientific field of clinical practice which is grounded in infectious diseases, epidemiology, and health systems science.
Which Came First?
The concept of infection prevention and control came first and can be traced back to the work of Ignaz Semmelweis, an obstetrician in the 1800s who championed the importance of handwashing to prevent puerperal (childbirth) fever and the associated high incidence of mortality. Unfortunately, as history asserts, Semmelweis was ridiculed and his germ theory was dismissed.3 The current-day profession of IP&C is instead based on the work of 19th century scientists such as Pasteur, Lister and Koch, and was recognized as a specialty almost a century later in the 1970s. In its early decades, the evolving specialty was led by registered nurses who still remain the single largest group of clinicians within the profession.4 Once assumed to be inevitable, clinical research in the area of healthcare-associated infections (HAIs) has proven that a significant percentage are preventable.
One of the first studies to prove this was the CDC’s Study on the Efficacy of Nosocomial Infection Control (SENIC) published in 1985 which estimated that 30 percent to 50 percent of HAIs are preventable with effective infection surveillance and control programs.5 Over the last 50 years, IP&C has evolved and expanded to become a rigorous, complex and essential clinical profession, often not understood by hospital administrators who tend to position these professionals subordinately to the non-clinical departments of quality or patient safety. In hospitals where they report directly to the C-suite (chief executive officer, chief medical officer, etc.), they are afforded the support and authority to execute their programs expediently, thereby reducing patient morbidity and mortality.6 When this is not the case, there is often associated under-market compensation, which in addition to the increasing incidence of retiring experienced IP&C professionals, and the ever increasing burden of data reporting, is resulting in an ongoing exodus of IP&C professionals nationwide.4 In response, the field of IP&C has opened its doors to individuals with public health and laboratory science backgrounds. However, there is no replacement for the registered nurse who has the training and knowledge of the clinical pathophysiology of infection and the physiological details that reveal the dynamic causes of sentinel HAI and outbreaks.7
The concept of quality improvement in healthcare was introduced in the early 1900s by Ernest Codman, a surgeon who first pioneered the creation of hospital standards and strategies to assess healthcare outcomes. The modern quality movement has since transformed to include creating practice guidelines in addition to ongoing comprehensive assessment of hospitals and healthcare providers.8 The current day hospital quality departments are typically well-resourced and well-positioned, often reporting directly to the C-suite.9
The concept of patient safety in healthcare was sparked in the late 1990s, with publication of the Institute of Medicine (IOM) report, “To Err is Human.” This report estimated that as many as 98,000 patients die from preventable errors in U.S. hospitals annually, a statistic which immediately garnered public and legislative attention.10 Local and national efforts subsequently began to focus on categories of preventable error such as pressure ulcers, patient falls and medication administration errors. This also rapidly led to development of new patient safety certifications, courses and positions in healthcare. This department is typically now well-resourced and positioned, often reporting directly to the C-suite.11
The departments of quality, patient safety and IP&C all support the overarching goal of optimizing patient outcomes in healthcare. Quality and patient safety use social theories to address improvement in a broad range of areas such as physician competence, medication administration, patient falls and pressure ulcer prevention. Infection prevention is a clinical science-based profession which stands parallel but equal to these non-clinical departments. Organizational charts should reflect (at a minimum) equivalence in authority for the three departments to ensure optimally expeditious interventions for reduction of patient morbidity and mortality associated with HAIs.
References
1. Johns Hopkins Medicine Armstrong Institute: https://www.hopkinsmedicine.org/armstrong_institute/_files/patient%20safety%20and%20quality%20improvement%20project%20tools/spirit_toolkit/the_differences_and_similarities_between_patient_safety_quality_improvement.pdf accessed January 14, 2021.
2. World Health Organization: https://www.who.int/gpsc/ipc/en/ accessed January 14, 2021.
3. Marjoua Y, Bozic KJ. Brief history of quality movement in U.S. healthcare. Curr Rev Musculoskelet Med. 2012;5(4):265-273. doi:10.1007/s12178-012-9137-8.
4. Hanchett M. The Infection Control Nurse: Approaching the End of an Era. Infection Control Today. Aug. 31, 2015.https://www.infectioncontroltoday.com/view/infection-control-nurse-approaching-end-era
5. Hughes JM. Study on the efficacy of nosocomial infection control (SENIC Project): results and implications for the future. Chemotherapy. 1988;34(6):553-61. doi: 10.1159/000238624. PMID: 3243099.
6. Barnes S, Spencer M. Reliable Design of IP Programs. Infection Control Today. Sept. 11, 2015.
7. Cox JL, Simpson MD. Microbiology Education and Infection Control Competency: Offering a New Perspective. J Microbiol Biol Educ. 2018;19(2):19.2.71. June 29, 2018. doi:10.1128/jmbe.v19i2.1475.
8. Luce JM, Bindman AB, Lee PR. A brief history of health care quality assessment and improvement in the United States. West J Med. 1994 Mar;160(3):263-8. PMID: 8191769; PMCID: PMC1022402.
9. Salary.com https://www.salary.com/research/job-description/benchmark/quality-management-director-healthcare-job-description#:~:text=Being%20a%20Quality%20Management%20Director,Typically%20reports%20to%20top%20management.
10. Lark ME, Kirkpatrick K, Chung KC. Patient Safety Movement: History and Future Directions. J Hand Surg Am. 2018;43(2):174-178. doi:10.1016/j.jhsa.2017.11.006
11. Institute for Healthcare Improvement: http://www.ihi.org/resources/Pages/Changes/DesignateaPatientSafetyOfficer.aspx
Sharps Injuries in 2020: The Year to Learn from the Past, Draw from the Present, and Improve the Future of Worker Safety in Healthcare
By Amber Hogan Mitchell, DrPH, MPH, CPH
This column originally appeared in the January 2021 issue of Healthcare Hygiene magazine.
Reprinted with permission from ORsafe.org
November 2020 marked 20 years since the passage of the Needlestick Safety and Prevention Act (NSPA). The Act required the Occupational Safety and Health Administration (OSHA) to update its 1991 Bloodborne Pathogens Standard to include new protections for workers facing exposures to blood, body fluids, and other potentially infectious materials.
At the time, advocates for worker health and safety and preventing sharps injuries and needlesticks were so aligned that champions from multiple disciplines and backgrounds came together seamlessly to fight for stricter policies that addressed safer conditions in healthcare. The Act was passed unanimously by Congress Nov. 6, 2020. Clinicians affected by injuries, worker safety and health advocates, manufacturers, unions, law makers, and regulators came together to improve the coverage the standard offered to workers.
At a high level, this meant inclusion of more specific requirements for:
- The evaluation and use of engineering controls, including safer medical devices like needleless systems and “sharps with engineered sharps injury protections” (devices with sharps injury prevention features).
- The inclusion of frontline non-managerial employees in the identification, evaluation, and selection of engineering controls and work practices.
- Establishing and maintaining a sharps injury log (beyond what is required by the OSHA Recordkeeping Rule).
On a more facility-specific level, this meant then and still means now that employers – despite being faced with competing safety and quality initiatives -- do not lose sight of identifying where injuries are occurring, during what procedures, and with what devices or practices. This includes using the Sharps Injury Log as an evergreen tool to direct campaigns and controls that prevent future injuries and learn from past ones.
Over the 20 years when incident data is compared at the facility-level to the national or regional levels, data tracks true to bigger surveillance systems that collect and report sharps injuries to the public, including the Exposure Prevention Information Network (EPINet®) from the International Safety Center and the Sharps Injury Surveillance System (SISS) from the Massachusetts Department of Public Health (MA DPH). As a nation, we saw great strides for decreasing incidence of injuries from blood collection devices, IV catheters, and lancets. Technologies got better, safer and more intuitive and the tide changed for the benefit of exposure prevention.
Today, however, reported incidents of injuries from hypodermic syringes, suture needles, and scalpel blades continue to be unacceptably high. In fact, according to both EPINet and MA SISS, these three device categories represent the highest numbers of injuries compared to all other device types in recent reporting years. They remain the devices that need greater attention relative to identifying and selecting better, safer alternatives and effective work practice controls like “safety” feature activation, no hands passing, and safe disposal.
Key Questions Looking Forward
In 2021, will we see percentages of device types change because of the pandemic? If we don’t manage the delivery of SARS-CoV-2 vaccines using devices with sharps injury prevention features will we see a drastic increase of injuries from hypodermic needles?
How might these reported incidents change in a pandemic age where there is more focus on keeping adequate stock of personal protective equipment like respirators and less on engineering controls for sharps injury prevention?
Given overcrowding and careful management of capacity available for patients suffering with COVID-19 or flu and protecting workers from airborne infectious disease, might focus on preventing exposures to bloodborne pathogens falter?
Given more focus than ever on worker health and safety in healthcare due to the global pandemic are we at continued risk of compromising worker safety for patient safety or will the tide change? Will we continue to sacrifice, overwork, and under-resource our healthcare workforce or will the pandemic improve conditions?
2020 Reflection
Yes, it has been 20 years since the passage of the NSPA and we celebrate that momentous occasion and yes, we are working through a global pandemic and we hope to see the light at the end of the tunnel in the coming months. 2020 has been wrought with ups and downs, challenges and opportunities, successes and failures and we must use what we have learned to make healthcare better and safer for those who work in it and those who access it.
Focus on COVID must not mean that we lose focus on sharps injuries that we can see and know how to prevent. Focus on PPE to prevent infectious disease exposures must not mean that we ignore what we know about the industrial hygiene hierarchy of controls and lose focus on the effectiveness of engineering controls and safe work practices. These include not only the use of devices with sharps injury prevention features like retracting needles and blades and suture-alternatives for skin closure, but safety feature activation, and responsible and safe disposal. This also includes shining a light on facilities, advocates, and manufacturers that get it right, work together, stay the course, and collaborate on developing and using the best devices to ensure the highest quality outcomes for workers and patients alike.
Amber Hogan Mitchell, DrPH, MPH, CPH, is currently president and executive director of the International Safety Center as well as the immediate past chair of the Occupational Health and Safety section of the American Public Health Association (APHA). Mitchell's career has been focused on public health and occupational safety and health related to preventing infectious disease. She is also a senior science adviser for the NIEHS Worker Training Program for COVID-19 response. Mitchell holds an adjunct faculty position at the University of Maryland School of Medicine Department of Environmental and Occupational Medicine. Mitchell has a new book available called Preventing Occupational Exposure to Infectious Disease in Health Care: A Practical Guide.
Herd Immunity and COVID-19
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the December 2020 issue of Healthcare Hygiene magazine.
When a large percentage of a community is immune to an infectious disease, ongoing transmission of the disease is unlikely. This state is termed herd immunity, and it can be achieved through vaccination (vaccine induced immunity) and through infection with the disease (natural immunity). It is important to note that by reducing viral transmission, herd immunity provides protection to those who cannot be vaccinated such as newborns, and immunocompromised individuals.1
The number of people required to be immune to achieve herd immunity is based on the contagiousness of the disease. Those infectious diseases that spread easily, such as measles, require a higher number of immune individuals in a community to reach herd immunity. COVID-19 is also a very contagious disease. Experts estimate that in the United States, more than 200 million people (70 percent of the population) would have to recover from COVID-19 and develop effective natural immunity in order to achieve herd immunity, thereby halting the epidemic.2
There are a lot of unknowns with COVID-19 which add complications to the goal of achieving herd immunity. Most importantly we do not have a vaccine and may not for some time. Additionally, we do not know if immunity is always conferred by COVID-19 infection, nor which people develop natural immunity after infection, and how long immunity might last. Even once a vaccine is developed, there are variables that will impact its effect on the pandemic. These include how effective the vaccine is, how many vaccine doses will be available for distribution, and how many people are willing to get vaccinated. This last variable is anticipated to be a significant challenge given the number of people who refuse to receive the annual influenza vaccination. And of course, even with a vaccine, until herd immunity is achieved, cases introduced by those traveling from outside of the U.S. will contribute to the ongoing US COVID-19 epidemic.3
Given the lack of a unified national response to the COVID-19 pandemic in the U.S., a wide spectrum of approaches can be seen relative to prevention tactics from state to state. This has also resulted in the highest case and death count globally, and dissent among various experts regarding the best method of achieving herd immunity.4 On the one hand, there is the Great Barrington Declaration. This is a statement written by three public health experts, which supports achieving herd immunity by allowing COVID-19 to spread in the young and healthy population where it is less likely to be deadly. The three experts suggest that the current COVID-19 prevention measures are resulting in greater harms than the pandemic, including economic instability, lower childhood vaccination rates, fewer health screenings, and deteriorating mental health.5
This Declaration was denounced by the John Snow Memorandum, which was published in response and signed by 6,900 scientists and health experts.6 The memorandum recommends that restrictions (not lock down) should be continued, in addition to social and economic programs and vaccine development. These experts explain that the primary concern with permitting spread of COVID-19 is that it would lead to the death of one to two million people, without necessarily speeding up society’s return to business as usual. In addition, if so many people become sick with COVID-19 at one time, hospitals would become quickly overwhelmed.
Many medical professionals use the term “herd protection” instead of herd immunity, because the phenomenon doesn’t actually result in immunity to the virus. Instead it reduces the risk that non-immune people will come into contact with the virus. Applying the concept of herd immunity through community spread of SARS CoV2, would be based on the unproven assumption that anyone who survives an infection will become immune. From studies of the pandemic to date, it does seem that some kind of immunity seems to follow infection, but it is unclear how long and for which people this occurs. And we don’t yet have a definitive method to measure immunity to SARS CoV2 virus.7
In the absence of a national approach to the pandemic, it may be prudent to look to the clinical professionals for guidance (John Snow Memorandum), versus those in public health sector (the Barrington Declaration) if the two are not in alignment. Clinical experts not only have training and experience regarding infectious diseases they are also at the front lines, observing first-hand what is happening with the pandemic. As concluded by the John Snow Memorandum, herd immunity for COVID-19 cannot safely be achieved with natural immunity alone but must be supplemented with vaccine induced immunity once an effective vaccine is widely available. In the meantime, local governments will continue to determine what restrictions on business are needed, supplementing social distancing and mask/face coverings, to contain the pandemic.
References:
1. CDC. https://www.cdc.gov/vaccines/vac-gen/immunity-types.htm Last accessed November 3, 2020.
2. Mayo. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/herd-immunity-and-coronavirus/art-20486808 Accessed Nov, 3, 2020.
3. The Lancet. https://doi.org/10.1016/S0140-6736(20)32153-x Accessed Nov. 3, 2020.
4. Altman D. Understanding the US failure on coronavirus. BMJ 2020; 370:m3417. DOI: https://doi.org/10.1136/bmj.m3417 (Published Sept. 14, 2020).
5. Great Barrington Declaration. https://gbdeclaration.org/ Accessed Nov, 3, 2020.
6. John Snow Memorandum. https://www.johnsnowmemo.com/ Accessed Nov. 3, 2020.
7. Aschwanden C. The false promise of herd immunity for COVID-19. Nature online; Oct. 21, 2020. DOI: https://doi.org/10.1038/d41586-020-02948-4 Accessed Nov. 3, 2020.
COVID-19 Lessons Learned at Nine Months
By Phenelle Segal, RN, CIC, FAPIC
Editor's note: This column originally appeared in the November 2020 issue of Healthcare Hygiene magazine.
November 2020 signifies an unprecedented nine months into the COVID-19 pandemic that took the nation by surprise and resulted in a tragedy on many levels. The intensity and speed with which this virus entered the United States turned healthcare facilities upside down across the continuum of care. Acute care hospitals were unprepared with inadequate supplies of personal protective equipment (PPE), disinfectants and equipment, thereby creating challenges of unprecedented proportions. Hospital beds were filling at alarming rates and several hospitals were turning non-clinical areas into wards or units. Makeshift hospitals were being erected in some cities and staff shortages were extreme.
Long-term care and outpatient facilities including doctor’s offices and clinics were unable to obtain supplies due to demand outweighing supply and this resulted in a state of chaos. Nursing homes and other long-term care settings in many regions were hit very hard with facility outbreaks and many elderly residents died. Lack of preparation was not necessarily the fault of the individual facilities or offices and practices, but rather, akin to the “big earthquake.” Unpredictable until it happens and particularly with such intensity.
Well into the pandemic, due to the diligence of the healthcare industry, Food and Drug Administration (FDA), Environmental Protection Agency (EPA) the Occupational Safety and Health Administration (OSHA) and private organizations as well as supply companies, obstacles have been approached aggressively. Depending on the location of facilities and the number of COVID-19 cases, supplies including personal protective equipment or PPE have become more available, albeit an ongoing shortage of N95 masks for some acute care and non-acute care settings. Many facilities have been able to revert to conventional capacity as per the Centers for Disease Control and Prevention (CDC) guidance developed earlier in the pandemic for optimizing PPE. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html.
Surge Capacity
A critical component of the ability to respond to large-scale disasters is surge capacity. Surge capacity is defined as “a healthcare system’s ability to expand quickly beyond normal services to meet an increased demand for medical care” or “ the ability to expand care capabilities in response to sudden or more prolonged demand.”
Emergency preparedness and planning in response to the terrorist attack on 9/11 was ramped up and beginning in 2003, The Joint Commission required all acute-care hospitals to develop a written plan based on facility hazard vulnerability assessments. In 2004, The Agency for Healthcare Research and Quality (AHRQ) expanded its Bioterrorism Planning and Response research and focused on ways to expand bed capacity in hospitals and develop surge requirements. In addition, in 2008 the U.S. Department of Health and Human Services (HHS) and the Centers for Disease Control and Prevention (CDC) were included in the emphasis on emergency preparedness and planning, with the Department of Homeland Security funding initiatives.
Effective emergency preparedness in healthcare requires planning for large-scale situations that affect many people. These events include terrorist attacks resulting in multi-casualty trauma, chemical, biological and radioactive events. Infectious disease epidemics and pandemics between 2003 and prior to COVID-19 included severe acute respiratory syndrome (SARS), Middle Eastern Respiratory Syndrome (MERS), H1N1 flu and Ebola virus. Each event includes subtle differences in the type of capacity needed, but general principles apply.
For at least two decades, hospitals have provided a written plan evaluating their hazard risks, resources, and an idea of their general ability to handle a surge including highly transmissible infectious agents. Prior to COVID-19, long-term care facilities (LTCFs) which includes nursing homes, assisted living facilities, and rehabilitation centers were expected to address surge capacity for the purpose of being available either for acute-care patients or for patients who were discharged early from traditional acute-care facilities to make beds available for additional acute care victims needing hospitalization.
Nursing homes had plans in place for some public health emergencies, but many had not done planning specific to pandemics such as COVID-19. Natural disaster plans were available such as those for wildfires and earthquakes. Planning was also depended on facility location and state requirements.
In planning for emergencies, concerns in nursing homes included caring for special patient populations during an emergency. Concerns about staffing in an emergency were seen across the board and staff were reluctant to leave their families. Lack of adequate amounts of medications and medical supplies as well as storage space for such were of concern too. Many nursing homes across the nation were willing to accept residents from area hospitals but concerns regarding the patient acuity levels and subsequent staffing and building capacity issues were of concern.
Key Components of Surge Capacity
The key components of surge capacity are known as “4 S’s” and include Staff, Stuff, Structure and Systems.
Staff
Key personnel include clinical staff such as physicians, nurses, respiratory therapists, pharmacists and technicians. Support staff such as environmental services, physical plant services, security services and clerical workers are indispensable during a pandemic. Intensive care units and emergency departments are two areas that require a tremendous number of staff. Repurposing of staff may be required including retired clinical personnel or those with expired professional licenses.
Staff shortages due to long shifts and physical exhaustion, PPE burnout due to mandatory use, emotional burnout, resignations, inability to work due to childcare or elder care needs and ill personnel with several deaths are expected.
Stuff
Surge capacity stuff includes durable equipment such as ventilators, oxygen masks, oximeters, defibrillators, intravenous (IV) pumps, blood glucose and INR monitors, cardiac monitors, hospital beds and wheelchairs. In addition, patient supplies include medications, IV catheters and fluids, oxygen, syringes, sutures, sterile dressings and PPE. Shortages are expected when a pandemic of epic proportions takes over a nation and unanticipated supplies are needed.
Structure
Hospitals will need to be reconfigured including utilizing rooms that are not typically patient care rooms, converting positive into negative pressure rooms, use of the operating rooms with anesthesia machines for critical patients and rearranging the emergency department. Outpatient clinics could be used as a satellite emergency department. Designating COVID-19 units and COVID-free units is essential. Staff should be dedicated to both. During epic pandemics, these needs will most likely be impossible to fulfill, especially at the beginning of the wave.
Systems
Systems include a clear chain of command for different activities. This chain of command should have a commander available day and night and be a good communicator with an effective communication strategy inside and outside the facility. The ability to share information among staff, the media, patients or residents and families is essential. Systems include written policies, procedures and protocols as well as education of personnel. Systems also include designating personnel to be responsible for “stuff” including PPE and reporting issues to the chain of command.
Summary
COVID-19 challenges during the past nine months have forever changed the focus of emergency planning. Surge capacity and planning for future disasters will require ongoing dedication and determination across the continuum of healthcare. The combination of tremendous teamwork, flexibility, creativity and coping skills inherent in healthcare professionals is evident several months into the disaster and as we prepare for the “winter wave” combined with influenza, facilities including acute, long-term and outpatient care are ready to: face existing and new challenges with strength and determination.
Phenelle Segal, RN, CIC, FAPIC, is president of Infection Control Consulting Services.
References:
1. Surge Capacity: Disaster Medicine. 2006: 193–202. Published online 2009 May15. doi: 10.1016/B978-0-323-03253-7.50035-2
2. Exploring the Concept of Surge Capacity" OJIN: The Online Journal of Issues in Nursing; Vol. 14 No.2. DOI: 10.3912/OJIN.Vol14No02PPT03
3. How to Surge to Face the SARS-CoV-2 Outbreak: Lessons learned from Lombardy, Italy. Disaster Med Public Health Prep 2020 Apr 1: 1-3. doi: 10.1017/dmp.2020.64
Leading the Way to Zero: Integrating What We’ve Learned to Create the “New Normal”
By Sylvia Garcia, MBA, RN, CIC
Editor's note: This column originally appeared in the October 2020 issue of Healthcare Hygiene magazine.
On Sept. 14, 2020, The Institute for Healthcare Improvement’s National Steering Committee for Patient Safety released Safer Together: A National Action Plan to Advance Patient Safety. This plan emphasizes that “…safety requires a shift from reactive, piecemeal interventions to a proactive strategy in which risks are anticipated and system-wide safety processes are established and applied…”
Anticipating risks and implementing safety processes are an infection preventionist’s “bread and butter.” We are trained to systematically apply models to explain and prevent transmission of infections within our organizations. The chain of infection is one model and another is the Hierarchy of Controls. The chain of infection identifies the route of transmission as the weakest link in the chain. If we can break that link, we can prevent transmission. n the Hierarchy of Controls, the most effective methods are at the top: elimination and substitution, and the least effective methods are at the bottom: administrative controls and personal protective equipment. Using what was known about COVID-19 transmission, initial reaction to COVID-19 focused on an integrated response to prevent transmission of COVID-19 which strived to:
• Eliminate the hazard and conserve resources by stopping elective procedures and limiting or barring visitors
• Substitute processes such as telehealth to provide care
• Install engineering control such as physical barriers at reception points
• Implement administrative controls such as screening everyone entering the facility for symptoms
• Provide personal protective equipment (PPE) to our frontline workers.
As cases increased around the world and transmission from asymptomatic cases was identified, organizations adjusted their approaches. Many people started to wear face masks and respirators out in public and in healthcare settings causing confusion and controversy. With dire PPE shortages in some parts of the United States, public officials emphasized that medical face masks and respirators should be reserved as PPE for those providing care to patients. In an effort to conserve scarce resources, some organizations restricted use of medical and surgical masks and respirators to those providing direct care to patients with known or suspected COVID-19.
Face coverings (masks made of cloth) were suggested as an alternative that would conserve resources for frontline workers. Initial information indicated that face coverings would not protect the wearer but could protect others. Now, increasing evidence concludes that universal masking and use of face coverings can protect the wearer and others from COVID-19.
The effect of COVID-19 prevention methods on influenza in the southern hemisphere countries offers hope for a controlled influenza season. Data on influenza from Australia, Chile, and South Africa shows very low influenza activity during June thru August 2020, the months that reflect typical Southern Hemisphere influenza season. Summer circulation of influenza in the United States is at a historical low.
There is no “magic bullet” that, by itself, will prevent transmission of respiratory viruses, including COVID-19 and influenza. Historically, we have focused on respiratory etiquette - encouraging patients to cover their cough and when possible wear a mask if they have respiratory symptoms. Despite improving influenza vaccination rates and implementation of respiratory etiquette, health care organizations have continued to identify health care associated transmission to patients and staff. As we move into the “new normal” of COVID-19 and face the start of influenza season, we need to use information we have learned from our experiences with COVID-19 to protect our patients, visitors, and staff. The CDC estimates that 3 to 8 percent of the U.S. population contracts influenza each year. Like COVID-19, influenza is transmissible before symptoms develop.
With information evolving on the role of microdroplets and ventilation, specifically air exchanges, in transmission of COVID-19, health care organizations need to plan for the coming winter. Rigorous implementation of COVID-19 prevention strategies including universal masking, social distancing, hand hygiene and surface disinfection can control COVID-19. When paired with influenza vaccination, these strategies may also lead to an influenza season with low transmission and provide information that could lead to new proactive strategies to prevent transmission respiratory viruses.
Infection preventionists must be able to integrate evolving information to make convincing recommendations for prevention to their leadership teams. Although work on the National Action Plan began before COVID-19, its release is timely as the core principles and recommendations are extremely relevant to the pandemic. Ultimately, the decision on which prevention strategies to implement and when they should be implemented is in the collective hands of health care organizational leaders. Even with state mandates, health care organizations can choose to rigorously educate and enforce implementation, or they can, instead, put up signs and call it a day. Ideally, it is the hope of infection preventionists, like me, that healthcare organizations will lead the way to zero harm by using the valuable information we are learning during this pandemic.
Sylvia Garcia, MBA, RN, CIC, is director of infection prevention and control within the of Division of Healthcare Improvement at the Joint Commission.
References:
1. National Steering Committee for Patient Safety. Safer Together: A National Action Plan to Advance Patient Safety. Boston, Massachusetts: Institute for Healthcare Improvement; 2020. Available at: www.ihi.org/SafetyActionPlan
2. Centers for Disease Control and Prevention. Principles of Epidemiology in Public Health Practice, Third Edition. Available at https://www.cdc.gov/csels/dsepd/ss1978/index.html Accessed September 29, 2020.
3. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. Workplace Safety and Health Topics. Hierarchy of Controls. Available at https://www.cdc.gov/niosh/topics/hierarchy/default.html Accessed September 28, 2020
4. Occupational Safety and Health. Recommended Practices for Safety and Health Programs. Hazard Prevention and Control. Available at https://www.osha.gov/shpguidelines/hazard-prevention.html Accessed September 28, 2020.
5. Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. Published online July 14, 2020. doi:10.1001/jama.2020.12897
6. Hendrix MJ, Walde C, Findley K, Trotman R. Absence of Apparent Transmission of SARS-CoV-2 from Two Stylists After Exposure at a Hair Salon with a Universal Face Covering Policy - Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep 2020;69:930-932. DOI:http://dx.doi.org/10.15585/mmwr.mm6928e2external icon
7. Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased Influenza Activity During the COVID-19 Pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1305–1309. DOI: http://dx.doi.org/10.15585/mmwr.mm6937a6
8. Centers for Disease Control and Prevention. Key Facts About Influenza (Flu) Available at https://www.cdc.gov/flu/about/keyfacts.htm Accessed on September 29, 2020.9. Centers for Disease Control and Prevention. How Flu Spreads. Available at https://www.cdc.gov/flu/about/disease/spread.htm#:~:text=When%20Flu%20Spreads,7%20days%20after%20becoming%20sick. Accessed September 29, 2020.
Beyond Bundles: Resources to Support IP Departments During the Pandemic
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the September 2020 issue of Healthcare Hygiene magazine.
During the current COVID-19 pandemic, infection prevention department (IPD) resources have been dramatically impacted by efforts required for containment of the virus. There is, consequently, less focus on prevention of healthcare-associated infections (HAIs). This diversion of focus is creating risk for patients according to clinicians at the Virginia Commonwealth University Health System. Their Twitter survey in April revealed that the majority of more than 200 respondents reported that the pandemic was consuming 75 percent of IPD time and resources.1
The report from the authors of a recent clinical paper makes similar observations, based on the experience of several of the hospitals in New York and Missouri.2 They suggest that the COVID-19 pandemic will significantly impact the rate of central line-associated bloodstream infections (CLABSIs), which have been observed to increase more than 300 percent in two hospitals over the past 15 months. The authors theorize that this may be due to smaller denominators as a result of fewer elective procedures, decreases in hospital census, as well as an increase in high-risk patients. In addition, proning critically ill COVID-19 patients could potentially cause disruption of central-line dressings.2 Also adding to the risk in some locations, intravenous (IV) tubing is being extended so that IV pumps can be kept outside of patient rooms. This creates the potential risk of contamination of tubing when in contact with floors.3
This is truly concerning, given that even prior to the pandemic, zero preventable HAI had not been achieved and/or sustained in many U.S. healthcare facilities.4 In most hospitals, a bundle of standard measures (i.e., supported by category 1 level evidence, defined as at least one properly designed randomized controlled trial) is the first line approach for prevention of all categories of HAI. When zero preventable infections are not achieved with a bundle of standard measures, one or more plus measures are often added (defined as supported by less than category 1 level evidence such as cohort or case control studies and expert opinion). For example, a standard bundle measure for prevention of surgical site infections (SSIs) is controlling serum glucose. An example of a plus measure for prevention of SSIs is pre-operative nasal decolonization. The number and type of plus measures is dynamic and always changing as studies are completed on emerging technologies and evidence is made available. Keeping pace with this dynamic body of knowledge is time-consuming. Consequently, in one large multi-hospital system, the national infection prevention program leader developed a Beyond Bundles Plus Measures HAI Prevention Toolkit to help the organization’s infection prevention staff keep pace with the constantly evolving infection prevention measures (products and practices) and associated evidence of efficacy.
Beyond Bundles Plus Measures HAI Prevention Toolkit
This 48-page document includes evidence summaries for all products and practices included, and is updated every two years. It is organized in chapters by infection type as follows: CAUTI (catheter associated urinary tract infection), CDI (Clostridium difficile infection), CRBSI (catheter related bloodstream infection which includes prevention of both CLABSI and peripheral bloodstream infections), HAP (non-ventilator associated hospital acquired pneumonia), MDRO (multi-drug resistant organism infections), VAP (ventilator associated pneumonia) and SSI. Each chapter begins with a list of standard bundle measures. Following the standard measures in each chapter, a table lists the plus measures designed to prevent that particular type of HAI. The plus measures are identified during deep review of clinical journals, attendance of professional conferences, continuing education courses, and networking with clinical experts and industry partners by the author. The collection is not intended to be exhaustive, as it is limited by the author’s due diligence in the collection and review of this clinical information. Vendors have been invited to share evidence, and product names are included when evidence of efficacy has been made available. So, although the toolkit includes product names, it does not seek to promote any product or company.
Following each plus measure there is an embedded document containing an evidence summary, which is also updated every two years. Product order information is provided in the form of links and text, as well as tools supporting implementation of the product or practice, such as video clips and embedded checklists and guidelines. The toolkit is available for download in full, and also by individual chapter, in open-access format and has been publicized widely including via social media. https://www.zeroinfections.org/toolkits.html
Especially today, when faced with the COVID-19 pandemic, infection preventionists are challenged with competing priorities. This open-access toolkit, offers a no cost aid for IP departments which supports the resource intensive task of reviewing the emerging evidence of efficacy associated with plus infection prevention measures. Unless zero preventable infections have been achieved with standard bundle measures, this is an essential task in optimizing infection prevention programs.
References:
1. Stevens M, Doll M, Pryor R, Godbout E, Cooper K and Bearman G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect Control Hosp Epidemiol. 41(8), 946-947. 2020. doi:10.1017/ice.2020.141
2. McMullen KM, Smith BA, Rebmann T. Impact of SARS-CoV-2 on hospital-acquired infection rates in the United States: Predictions and early results. July 2, 2020. Am J Infect Control. 2020; S0196-6553(20)30634-9. doi:10.1016/j.ajic.2020.06.209
3. Institute for Safe Medication Practices: https://ismp.org/resources/clinical-experiences-keeping-infusion-pumps-outside-room-covid-19-patients
4. CDC HAI Progress Report: https://www.cdc.gov/hai/data/portal/progress-report.html
Navigating COVID-19’s Ongoing Challenges: A Perspective From the Front Lines of Infection Prevention
By Phenelle Segal RN, CIC, FAPIC
This column originally appeared in the August 2020 issue of Healthcare Hygiene magazine.
In a pre-COVID 19 world, August is the height of summer vacation for millions of Americans. People enjoy backyard barbecues, swimming parties, fun at the beach, traveling and organizing family reunions as the nation enjoys a much-needed reprieve from cold temperatures and long work hours. The summer of 2020 is very different as it continues to reveal a tumultuous and unprecedented pandemic. COVID-19 continues to follow the trajectory of an out of control respiratory-spread virus, that has the power to sicken and kill many Americans within a short period of time. Besides the tragic toll on human lives, COVID-19 continues to affect the economy and threatens healthcare facilities and workers with no end in sight. The ongoing challenges we face is evident including severe shortages of personal protective equipment (PPE) and disinfectant products among many others.
Ongoing Challenges in Healthcare Facilities
Over the course of five months, infection preventionists -- after planning and preparing as best as possible -- were unaware of the impact an out-of-control, highly transmissible respiratory virus could have on a system-wide basis. Prior pandemics including SARS, H1N1, MERS and Ebola revealed the need to stay on top of surge capacity plans in the event of a “COVID-19 catastrophe. However, in line with other natural disasters, we had no idea when it would strike, what type of disease would attack and how much of an impact it would have. We were always aware that our efforts to plan for the “big one,” may fall short of the needs as the unknown would deliver its punches. Decades of developing, implementing, and educating on “best practices” have abruptly halted as infection preventionists and healthcare educators scramble to prioritize and use best judgment, while guiding facilities across the continuum of care. The frustration in having to let go of routine practices is daunting, but infection preventionists must be flexible in an ever-changing environment. This article will address two ongoing critical challenges as we continue striving in a nontraditional fashion to strive for staff and patient safety.
Personal Protective Equipment (PPE) Shortage
Filtering face-piece respirators (FFRs) including but not limited to N95 respirator masks are critical items in the prevention of COVID-19 spread and other aerosol transmissible diseases. They remain in ongoing short supply throughout the nation. FFRs protect the user by filtering particles out of the air that is being breathed by the users. The National Institute for Occupational Safety and Health (NIOSH) the federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness has seven classes of FFRs approved with a ninety-five percent minimum level of filtration (95 percent). Masks that filter less than 95 percent of particles are not guaranteed to be as effective as those that filter 95 percent or more. NIOSH works in conjunction with the Occupational Health and Safety (OSHA) agency that regulates respiratory programs for healthcare workers.
N95 masks are the traditional FFR used in hospitals for healthcare personnel taking care of patients requiring airborne isolation. The most common use has been for patients with aerosol transmissible diseases including pulmonary tuberculosis (TB). They are manufactured and sold as “single use only” and until COVID-19, there was no shortage of these items.
In response to the increased demand for use as thousands of cases were occurring in the hot zones in March and April, the Food and Drug Administration (FDA) released Emergency Use Authorizations (EUAs) for companies that had developed a “mask reprocessing” system to decontaminate N95s for reuse. Only N95 masks can be decontaminated but is dependent on the manufacturer and products used. Some N95 masks are not compatible with reprocessing such as those made with cellulose. In addition, the Centers for Disease Control and Prevention (CDC) issued guidance for reuse and extended use of single use FFRs. To date, facilities are reprocessing N95 masks via authorized methods and strictly follow the manufacturer of the mask as well as the decontamination equipment’s instructions for use. These methods are primarily using hydrogen peroxide in various forms, but with limited numbers of reprocessing cycles (based on the type of equipment) before having to discard them.
Infection preventionists continue to work with facilities that cannot reprocess masks and one of the CDC recommendations for extending the “life of the mask” is to place them in a brown paper bag or other breathable container for at least 72 hours before wearing them again. Facilities are providing a limited number of N95 masks to employees at most risk. That includes healthcare workers caring directly for COVID-19 positive and those providing aerosol generating procedures (AGPs) such as anesthesiology personnel. Staff are wearing surgical masks over their N95 masks to prevent them from becoming decontaminated. Face shields are thought to provide some protection from becoming contaminated too. Reuse and disinfection techniques are neither simple, nor ideal, but at this juncture, the choices are limited. It is important to note that masks must be discarded if they are visibly soiled, damaged or become moist/wet as they will not function effectively.
OSHA (29 CFR 1910.134) requires a medical evaluation, fit-testing and training prior to use of N95 masks performed initially (before the employee is required to wear the N95) followed by annual fit testing prior to COVID-19. However, OSHA did provide Temporary Enforcement Guidance in response to COVID-19 and despite the temporary guidance, challenges with lack of availability of appropriate sizes for staff members and short supply of solution for the fit test kits continues. A self-administered seal check should be performed before donning the masks.
Additional FFRs have been authorized by NIOSH for use including but not limited to “Surgical N95 respirators” and Powered Air Purifying Respirators (PAPRs). PAPRs do not have to be fit tested. Lastly, hospitals have turned to reusable elastomeric non-powered air-purifying half facepiece (half mask) manufactured to be reused, which has distinct advantages. They also need a fit test prior to first use.
Disinfectant Product Shortage
Coronaviruses are enveloped viruses and hence are extremely easy to kill using the appropriate disinfectant. Novel coronaviruses are unable to achieve a viral claim in a short amount of time and usually companies take a year or more to complete testing. Due to the length of time it takes to achieve this claim, the U.S. developed a policy based on a hierarchy for companies, meaning that if a product is effective against “harder to kill viruses, it is likely to kill COVID-19”. Harder to kill viruses include the non-enveloped group including norovirus, poliovirus, rhinorvirus, feline and reovirus.
Shortly after the pandemic was recognized as a potential threat as well as the emphasis placed on the size of COVID-19 droplets and the ability to settle on surfaces, disinfectant products became difficult to obtain as the demand outweighed the supply. This included online and in-store purchases as well as manufacturers and distributors running out of product. In response to the importance of surface disinfection and product shortages, the Environmental Protection Agency (EPA) developed an extensive list of products shortly after COVID-19 was exponentially spreading. Known as the EPA’s “List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19), it is constantly updated, and new products are being added. Facilities are encouraged to check the list regularly. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19
Hospitals continue to face shortages of disinfectant wipes and liquid with no immediate “return to normal” for availability of supplies. Manufacturers are developing creative strategies to provide product to healthcare facilities as a priority. The public has very limited access to supplies on shelves, with a one per person limit in most stores. Online purchase of products are prioritized for healthcare facilities only and the public does not have access to them.
Extreme shortages are occurring in non-acute care-based healthcare facilities at a higher rate than acute care hospitals. The primary reason in the non-hospital- based facilities is due to the product manufacturers prioritizing distribution based on previous use. Acute care hospitals use disinfectants on a much larger scale than non-acute based facilities. Manufacturers are reviewing order history together with supply when determining which facilities receive products and the quantity allocated.
Five months into the pandemic, with record numbers of cases appearing in many states and no end in sight, infection prevention challenges will continue to arise. It is incumbent upon us as healthcare providers, to face these hard times with strength, skill and perseverance as we continue to work at providing the support, strength and structure to our colleagues and patients.
Phenelle Segal, RN, CIC, FAPIC, is president of Infection Control Consulting Services.
Under-Addressed Risk Factors for COVID-19 and Future Pandemics
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the July 2020 issue of Healthcare Hygiene magazine.
We have learned much about critical risks factors that lead to global transmission of novel respiratory viruses, including the current COVID-19 pandemic as well as those in the past. This must in turn, inform our preparedness for future pandemics. The risk factors include a lack of herd immunity for novel viruses, inadequate/untimely transnational communication and collaboration when novel virus is first identified, inadequate stores of personal protective equipment, viral qualities such contagiousness and modes of transmission (e.g., coughing, sneezing, face touching, and contact with contaminated surfaces). Especially during the current pandemic, there is tremendous focus placed on mitigation strategies for each of these risk factors. However, there are two additional risk factors which have received less attention during the current and past pandemics. These are: 1) the infection transmission risk posed by pre-school and school-aged children and 2) the risk posed by Asian mixed wild animal wet markets, both in introducing novel viruses as well as transmitting them.
The Role of Pre-School and School-Aged Children
Young children often play a significant role in transmission of respiratory viruses including pandemic influenza (flu) and the current novel coronavirus SARS CoV2 which causes COVID-19 infections.1 Given that respiratory viruses are found in the nose and throat, and children frequently touch their noses, eyes and mouths, share objects that have been put in their mouths, and have physical contact during play, these viruses are easily transmitted among children and with parents, teachers and caregivers. Transmission then continues with contacts of the parents, teachers and caregivers. Exacerbating the current pandemic is the lack of immunity to this novel virus in both adults and children. And even with seasonal flu, young children may not have pre-existing herd immunity.2
Containing the transmission of pandemic flu and other respiratory viruses including SARS CoV2, posed by young children within our global population, will require ongoing education of children, parents and caregivers. The education must include information and training regarding hand hygiene, face coverings, environmental disinfection and physical distancing. It must also include sufficient sourcing of supplies (e.g. masks/face coverings, hand sanitizer, environmental disinfectant) to ensure that these are ubiquitous. Adequate annual influenza vaccination of all three groups, children, parents, and teachers will continue to be critical, in addition to SARS CoV2/COVID-19 vaccination when it becomes available. Nasal antiseptics as part of daily hygiene for children, teachers and caregivers would be arguably an added advantage providing an extra layer of safety. This type of product has been used successfully in healthcare to reduce infections by bacteria residing in the nose.3 Studies are needed to determine if nasal antiseptics can similarly reduce the risk of respiratory viral infections as well, but it is a good bet since respiratory virus is more easily inactivated than bacteria.4 Temperature screening, mask wearing for middle school, and physical distancing strategies, such as smaller class sizes, with rotation of students between in person classrooms and distance learning strategies may also be important in reducing the contribution of respiratory viral transmission by young children.5 Read more here:6 https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html
The Role of Live Animal Wet Markets in Asia
Asian wet markets where live animals are sold have been proposed or confirmed as the source of a number of pandemics including Asian Flu 1957, Hong Kong Flu 1968, SARS CoV1 2003 and SARS CoV2 2019.7 These markets create a perfect environment for the introduction and transmission of novel viral pathogens, where wild animals of many types from all over the world are brought into close contact which would never happen in the natural world.
The animals in the market are packed together for days and sometimes longer and are often slaughtered in the market where blood and body fluids are generated, and many humans are gathered. This is where viral pathogens can be transmitted between species and then to humans. This is especially true mainland China where the markets have many kinds of animals – some wild, some domesticated and not always native. These animals are stressed in crowded captivity among humans resulting in reducing their natural immunity and facilitating viral transmission, and with genetic mixing, novel viral strains. There are many types of wet markets in Asia, only the type described posing the greatest risk. There also many challenges to reducing or eliminating them including culture and tradition.8 Read more at: https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html
The elimination of all wet markets would not be a practical or reasonable approach, which would be like prohibiting all farmers markets in the U.S. However, reducing or eliminating live animal wet markets in mainland China should be considered, given the morbidity and mortality resulting from the pandemics which continue to be generated from such markets.9-11
Sue Barnes, RN, CIC, FAPIC is an independent clinical consultant, Board certified in Infection Control and Prevention, a Fellow of APIC (FAPIC) and co-founder of the National Corporate IP Director Network. She currently provides marketing and clinical consultation to select industry partners who seek to support infection prevention with innovative products.
References:
1. Mimura S, Kamigaki T, Takahashi Y, Umenai T, Kudou M, Oshitani H. Role of Preschool and Primary School Children in Epidemics of Influenza A in a Local Community in Japan during Two Consecutive Seasons with A(H3N2) as a Predominant Subtype. PLoS One. 2015;10(5):e0125642. Published 2015 May 5. doi:10.1371/journal.pone.0125642
2. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010 Mar 27; 375(9720):1100-8. doi:10.1016/S0140-6736(09)62126-7.
3. Mullen A et al. Perioperative participation of orthopedic patients and surgical staff in a nasal decolonization intervention to reduce Staphylococcus spp surgical site infections. American Journal of Infection Control. 45 (2017) 554-6.
4. Osborne K. Viruses, bacteria and fungi. Virox animal health online. October 31, 2017. https://www.viroxanimalhealth.com/blog/viruses-and-bacteria-and-fungi-oh-mydont-let-vicious-viruses-beastly-bacteria-or-freaky-fungi-haunt-you accessed June 13, 2020.
5. United Nations Educational, Scientific and Cultural Organization (UNESCO) https://en.unesco.org/news/back-school-preparing-and-managing-reopening-schools accessed June 13, 2020.
6. CDC Considerations for Schools https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/schools.html accessed June 13, 2020.
7. Webster RG. Wet markets--a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363(9404):234‐236. doi:10.1016/S0140-6736(03)15329-9.
8. Woo PC, Lau SK, Yuen KY. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr Opin Infect Dis. 2006;19(5):401‐407. doi:10.1097/01.qco.0000244043.08264.fc.
9. Beaubien J. Why They're Called 'Wet Markets' — And What Health Risks They Might Pose. January 31, 2020. NPR online https://www.npr.org/sections/goatsandsoda/2020/01/31/800975655/why-theyre-called-wet-markets-and-what-health-risks-they-might-pose accessed June 13, 2020.
10. Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989 Nov; 63(11):4603-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC251093/ accessed June 13, 2020.
Infection Prevention Guidance for the Hotel Industry During COVID-19
By Carol McLay, DrPH, MPH, RN, CIC, FAPIC
This column originally appeared in the June 2020 issue of Healthcare Hygiene magazine.
The current COVID-19 outbreak is placing unique psychological stress points on the nation’s healthcare workers (HCWs). Previous research has shown that epidemics can cause severe psychological effects. A recent review of the mental health problems faced by HCWs during this pandemic suggests that HCWs are experiencing considerable stress, anxiety, depression and insomnia.1
Reasons for these adverse psychological outcomes range from excessive workload and work hours, making difficult decisions about how to conserve limited personal protective equipment (PPE) and prioritize treatment, anxiety over putting their families at risk for exposure to the virus, and confronting a mounting death rate.
With their extended work hours and close contact with COVID-19 patients, health care workers have expressed anxiety about safe accommodations. Some workers have long commutes and need a place close by to rest and regenerate. Many worry that they may expose their families to the virus when they return home.
Temporary housing options help to support the mental health needs of staff. Healthcare facilities are creating partnerships with local hotels, universities and rental properties to offer accommodation to healthcare workers.
Hotel rooms for healthcare workers are provided at no-cost through the Rooms for Responders program offered by Marriott Bonvoy, in collaboration with American Express and JPMorgan Chase.2 Hilton and American Express are donating up to 1 million hotel rooms to individual front-line medical professionals during the COVID-19 crisis.3
The American Hotel & Lodging Association (AHLA) has developed the “Hospitality for Hope Initiative” to enhance collaboration between the hotel industry and the health care community, first responders, and local communities.4 As part of the initiative, AHLA is working closely with the Department of Health and Human Services (HHS) to create a national database of hotel properties willing to provide temporary housing for emergency and healthcare workers. At the time of writing, more than 17,000 properties have agreed to participate, offering 2.3 million rooms to our healthcare heroes.
In preparation for hosting HCWs, hotel managers and staff should take precautions to improve guest and employee health and safety.
General guidelines include:
- Follow local/state public health recommendations
- Reinforce personal hygiene throughout your hotel
- Place signage and floor markers in lobby, elevator landings, restaurant areas and other communal areas displaying appropriate physical distancing and health and hygiene reminders.
- Provide hygiene materials such as hand sanitizer in strategic locations such as entrances, elevator landings, fitness centers, restaurants, ice and vending machines.
- Utilize disinfectants that are effective against SARS-CoV-2, the virus that causes COVID-19. (See the EPA’s List N: Disinfectants for Use Against SARS-CoV-2 for a list of approved disinfectants, at:https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2)
- Post a list of precautions that your property will be taking to ensure that both guests and employees will remain safe during this period.
- Consider housing HCWs on a separate wing or section of the hotel reserved for this workforce
Specific Guidelines for Procedural Implementation
Employees
• Consider requiring employees to wear a mask in public areas at all times.
• Consider screening all employees upon arrival to work. Screening questions include:
1. Do you have any flu-like symptoms such as fever, cough or shortness of breath?
2. Have you been around anyone that is positive for COVID-19?
If an employee answers yes to any of the above, have them return home and call their healthcare provider.
• Employees must sanitize hands upon entry of hotel and frequently during their shift.
• Provide employee education about COVID-19 fundamentals such as signs and symptoms, mode of transmission, and prevention strategies to reduce spread including importance of hand hygiene.
• Ensure communications are culturally and linguistically appropriate.
• Train staff to use disinfectants safely and correctly. Staff should wear gloves when cleaning. Follow the manufacturer’s recommendations for proper use of disinfectants; for example, if the product has a 30-second contact time, this means that surfaces must stay wet for 30 seconds to be effective.
• Clearly communicate leave policies for planned and unplanned absences; provide guidance and support for childcare and elder care.
• Implement policies to reduce the frequency and type of face-to-face contact among employees (e.g., hand shaking, shared work areas, break room); ensure employees maintain 6 feet of distance between other employees and guests.
• Closely monitor employee health. Reinforce personal hygiene and cough etiquette.
• Provide hand sanitizer stations and tissues to all employees and guests.
Guests
• Consider requiring all guests to wear a mask in public areas at all times.
• Encourage guests to sanitize hands on entry to hotel.
Beverage service
• No self-service, front desk staff to provide coffee/tea service upon request.
Guest rooms
• Request that all guests exit room while room is being cleaned.
• Thoroughly clean and disinfect all hard surfaces, pay particular attention to high touch surfaces (see section below on specific touch surfaces).
• Do not reuse cleaning clothes between rooms, use disposable cloths/wipes if possible
• Remove bed scarves from service.
• Wash bedspreads/comforters between each guest.
• Remove minibar snacks and beverages from guest rooms, have available upon request.
Public restrooms
• Clean and disinfect public restrooms on a frequent basis.
• Use disposable wipes if possible.
Business center
• Restrict access to ensure social distancing.
• Disinfect after each use, including wiping of keyboard and other high touch surfaces.
Fitness facility
• Follow local guidance and adhere to strict physical distancing and sanitation protocols.
• Ensure guests have access to disinfection wipes.
• Frequent disinfection.
Public laundry facility
• Restrict access to one guest/family at a time.
• Disinfect laundry room after each use.
Laundry
• Linen may become contaminated with the virus. Employees should handle contaminated linen as little as possible with minimal agitation.
• Wash items in accordance with the manufacturer’s instructions using the hottest appropriate water setting; dry items completely.
• Ensure disinfectant is added to laundry wash.
• Clean and disinfect hampers or other carts for transporting laundry.
Dining room
• Follow local/state/federal regulations.
• Provide Grab and Go or pre-wrapped options.
• No self-service food/beverage stations.
• Update floor plans to allow for table configurations no less than 6 feet in proximity to other tables.
Specific Touch Points
Lobby and common areas
• Check-in desk
• Door handles, push plates, hand railings
• Countertops
• Telephone and keypad
• Tables and chairs
• Trash receptacle touch points
• Elevator buttons
Public restrooms
• Door handles
• Sink faucets and toilet handles
• Soap dispenser handle
• Towel dispenser
• Baby changing station
• Trash receptacle touch points
Guest rooms
• Door handles
• Desk, tables, chair, lamps
• Dresser drawer handle
• Drapery pull handles
• Mini-bar, menu, coffee maker
• Light switches and thermometers
• Telephone and keypad, remote control, and alarm clock
• Television
• Make up mirror, hand dryer
• Trash receptacle touch points
• Handles to clothes cupboard, hangers, luggage rack
Ice/vending machines
• Frequent disinfection of door handles, keypads, buttons
It is critical to ensure the health and safety of this essential workforce and the hotel industry is uniquely positioned to support our HCWs who are on the frontlines of this public health crisis. Providing a safe, clean and comfortable hotel room to our exhausted workers after another grueling shift provides much needed physical and psychological support and safety. In addition, this provides hotels a means to keep their doors open and give back during this time of crisis.
By supporting and addressing the social needs of the healthcare workforce, hospitals continue to show their commitment and dedication to keeping Americans safe and healthy.
Carol McLay, DrPH, MPH, RN, CIC, FAPIC, is the CEO of Infection Control International and is a consultant in the fields of healthcare epidemiology, infection prevention and control, and public health.
References:
1. Spoorthy M.S, Pratapa SK and Mahant, S. (2020). Mental health problems faced by healthcare workers due to the COVID-19 pandemic-A review. Asian Journal of Psychiatry. 51, 102119. Advance online publication: https://doi.org/10.1016/j.ajp.2020.102119
2. Rooms for Responders. Available at: https://marriottcares.marriott.com
3. Hilton and American Express Program. Available at: https://newsroom.hilton.com/corporate/news/hilton-american-express-team-up-to-donate-rooms
4. Hospitality for Hope. Available at: https://www.ahla.com/ahlas-hospitality-hope-initiative
COVID-19: The Journey From Mitigation to the ‘New Normal’
By Phenelle Segal, RN, CIC, FAPIC
Editor's note: This column originally appeared in the May 2020 issue of Healthcare Hygiene magazine.
In early January, clinicians in the United States were alerted to cases of a respiratory illness occurring since late December 2019 in dozens of patients from Wuhan, China. Clinicians were told to closely evaluate patients with symptoms and a history of recent travel to and from the affected area. On Jan. 21, 2020 the Centers for Disease Control and Prevention (CDC) officially confirmed the first case of a novel coronavirus in the state of Washington; the patient had returned from Wuhan on Jan. 15 and presented to a medical facility there. Due to his history of travel and respiratory symptoms, a new coronavirus illness was suspected, and a Real time Reverse Transcription-Polymerase Chain Reaction (rRT-PCR) test was run and confirmed the medical center’s suspicion. Within a month from first hearing about the initial cases, the world began experiencing the unprecedent 2019 Novel Coronavirus (2019-nCoV) pandemic.
When first identified in Wuhan, the virus was thought to spread from animal to humans, with no evidence that it was spreading from human to human. Like previous coronavirus organisms, COVID-19 appeared to originate in a poultry and seafood market during the latter part of 2019. The source was unclear with bats and pangolins highly suspected. Upon reaching the U.S., it was becoming more evident that person-to-person spread was a concern in China, but its rate and ability to spread remained unclear.
Healthcare facilities, particularly acute-care hospitals were overrun by sick patients, many of them requiring intensive care treatment with or without the use of a ventilator. Very ill patients have had a prolonged clinical course and delayed discharge due to an unprecedented list of clinical conditions. The huge influx of patients resulted in a tremendous shortage of personal protective equipment (PPE) and ventilators. The shortages were dependent on the region and directly proportional to the number of cases. After several weeks of frenzied care provided to hundreds of thousands of ill patients, many healthcare workers were and continue to be stricken with COVID-19 and several deaths have occurred.
Initial Mitigation Steps
Since CDC first heard of a surge in cases in Wuhan, the agency began preparing as best as possible, aware of the fact that it was a matter of time before the U.S. would see an influx. In conjunction with the White House, the following steps were taken and several remain in place to date:
• Developed an alert system for healthcare providers from the beginning of January.
• Provided guidance to clinicians about signs and symptoms as they were identified from Wuhan, and requesting they be alert for a positive travel history to and from potentially infected countries.
• Provided viral testing guidance.
• Provided preliminarily guidance for the care of patients in the home who may develop COVID-19.
• Provided guidance for airport screening of passengers coming into several major international airports.
• Assisted with developing a diagnostic test to detect this virus in clinical specimens.
• Activated its Emergency Operations Center to prepare for future support to healthcare providers.
• Deployed a team to Washington state to begin contract tracing and other support.
• Ordered each state to issue executive orders to shut down non-essential businesses, public gatherings, sports events, entertainment and stay at home orders.
• Ordered outpatient healthcare providers to cease providing non-urgent/non-emergent services including elective surgeries.
• Implemented social distancing strategies to curb the spread from close contact.
• Banned hospitals and nursing homes from visitors.
• Issued guidance for healthcare facility employees, vendors and essential persons to universally mask while in the building.
• Suggested individual states and counties implement face coverings for the general public.
Ongoing Mitigation
• Guidance was and continues to be released at an accelerated rate for the community and healthcare industry.
• Ongoing updates from many sources were and continue to be very helpful in developing plans for healthcare facilities.
• CDC deployed additional personnel to “hot zones”.
• Conference calls for healthcare providers and community were set up and continue to take place.
• Guidance provided for agencies and companies developing additional tests including antibody tests.
• Guidance for companies and agencies reviewing and trialing medications to treat ill patients.
• Providing input for agencies and companies researching vaccine development.
Returning to the New Normal
Three months into the pandemic, the White House has introduced guidelines to reopen the country using a three-phased approach. Besides other industries, the first and second phase includes resuming outpatient and inpatient elective surgery respectively. Visitor bans will continue to be strictly upheld during phase one and for the most part phase two for hospitals and nursing homes. Every state will need to develop “reopening plans,” which is expected to be extremely challenging and will require a multi-disciplinary team approach.
Outpatient surgery centers are closed to elective procedures with urgent or emergent procedures allowed at the discretion of the medical providers. Elective procedures are on hold in hospitals too. During the ban of elective procedures, staff were responsible for developing initial plans for screening patients and physical distancing protocols. In addition, outpatient centers were asked to develop plans for possible conversion to COVID-19 bed use and anesthesia machines for the purpose of ventilating patients. PPE was to be preserved and in certain regions, sent to hospitals for front line staff to use during care of infected patients.
Roadmap for Resuming Elective Surgery After COVID-19 Pandemic
In late April, a joint statement was released by the American College of Surgeons, American Society of Anesthesiologists, Association of periOperative Registered Nurses and the American Hospital Association. The following is a list to guide surgery centers and hospitals for resuming procedures :
• Timing for Reopening of Elective Surgery – Reopening should be considered only after a sustained reduction in the rate of new COVID-19 cases in the relevant geographic area for at least 14 days.
• COVID-19 Testing within a Facility – Facilities should use available testing to protect staff and patient safety whenever possible and should implement a policy addressing requirements and frequency for patient and staff testing.
• Personal Protective Equipment – Facilities should not resume elective surgical procedures until they have adequate PPE and medical/surgical supplies appropriate to the number and type of procedures to be performed.
• Case Prioritization and Scheduling – Facilities should establish a prioritization policy committee consisting of surgery, anesthesia and nursing leadership to develop a prioritization strategy appropriate to the immediate patient needs.
• Post-COVID-19 Issues for the Five Phases of Surgical Care – Facilities should adopt policies addressing care issues specific to postponement of surgical scheduling related to COVID-19
• Collection and Management of Data – Facilities should reevaluate and reassess policies and procedures frequently, based on COVID-19 related data, resources, testing and other clinical information.
• COVID-related Safety and Risk Mitigation surrounding Second Wave – Facilities should have and implement a social distancing policy for staff, patients and patient visitors in non-restricted areas in the facility which meets then-current local and national recommendations for community isolation practices.
• Additional COVID-19 Related Issues including:
Healthcare worker well-being: post-traumatic stress, work hours.
Patient messaging and communication.
Case scheduling process.
Facility and OR/procedural safety for patients.
Preoperative testing process for COVID-19-positive and non-COVID-19-positive patients.
Environmental cleaning.
Prior to implementing the start-up of any invasive procedure, all areas should be terminally
cleaned according to evidence-based information.
In all areas along five phases of care (e.g. clinic, preoperative and OR/procedural areas,
workrooms, pathology-frozen, recovery room, patient areas, ICU, ventilators, scopes,
sterile processing, etc.)
Regulatory issues (The Joint Commission, CMS, CDC).
Operating/procedural rooms must meet engineering and Facility Guideline Institute standards for air exchanges.
Re-engineering, testing, and cleaning
Pandemics are like natural disasters; their timing and magnitude is unpredictable. COVID-19 arrived precipitously, spread rapidly and quickly overwhelmed the nation. History has proven that respiratory viruses don’t disappear and often linger for a few years or an effective vaccine is developed. H1N1 in 2009 lingered for approximately three years. A vaccine was developed and was introduced as a component of the annual flu. COVID-19 vaccine development has begun, but the outcomes remain unknown at this juncture.
The “new normal” is beginning to take shape. Facilities across the continuum of care are working through the challenges of realigning compromised infection prevention “best practices. Patient safety and prevention of transmission of hospital-acquired conditions, while temporarily disrupted, remains unchanged. Healthcare professionals have spent decades improving hand hygiene, disinfecting the environment, appropriate isolation of potentially transmissible patients and more. These “best practices” will require reeducation and training sooner than later as healthcare services resume.
Phenelle Segal, RN, CIC, FAPIC, is president of Infection Control Consulting Services.
The Role of Infection Preventionists in Antibiotic Stewardship Programs
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the April 2020 issue of Healthcare Hygiene magazine.
Since their introduction in the 1940s, antibiotics have greatly reduced illness and death from all types of infections caused by bacteria. However, overuse has led to development of bacterial resistance, making the drugs less effective and creating bacteria that is more difficult to treat. Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics, and 23,000 people die as a direct result of these infections.1 To promote the appropriate use of antibiotics, antibiotic/antimicrobial stewardship programs (ASP) have been implemented in hospitals nationwide, and are required by the Joint Commission and the Centers for Medicare and Medicaid.2 These programs are making progress in reducing resistance, and the incidence of infections caused by multidrug-resistant organisms. In addition, the appropriate use of antibiotics also serves to reduce the incidence of Clostridium difficile infections (C. diff), caused in part by the disruption of helpful intestinal bacteria.1
Various perspectives have been offered regarding the role of the infection preventionist (IP) in ASP. A 2019 paper published in AJIC on the subject suggests that “the absence of a clear role definition for IPs in ASPs is likely hindering IPs from contributing in consistent, meaningful ways.”3 This was written subsequent to publication of two key Association for Professionals in Infection Control and Epidemiology (APIC) documents, suggesting that there is still work to do to clarify the role of the IP in ASP. The updated APIC-Society for Healthcare Epidemiology of America (SHEA) position paper on the role of the IP in ASP, published in 2018, proposes the following ASP related functions for IPs: 4
1. Leadership commitment: Infection prevention and control (IPC) and antimicrobial stewardship (AS) program leaders must work together to align their programs, promoting communication and collaboration, and reducing the likelihood of redundant initiatives.
2. Action: IPs can leverage strong collegial relationships to influence and facilitate nursing’s supporting role in initiating antibiotic timeouts, performing antibiotic reconciliation during patient transitions of care, and educating patients and families.
3. Tracking: IPC programs perform surveillance for emerging pathogens and resistance patterns, as well as rapid response to every possible transmission.
4. Reporting: IPC programs are responsible for HAI surveillance and providing feedback of infection rates (e.g., multidrug-resistant organisms and Clostridioides difficile/CDI) and audit data (e.g., hand hygiene adherence) to clinicians and other stakeholders. CDI prevention is a high priority for IPC and AS programs, so sharing and disseminating antibiotic use and CDI infection rates is essential to prevention efforts.
5. Education: Some specific examples include providing education to frontline healthcare workers regarding the appropriate collection of urine cultures, cultures from endotracheal tubes, and indications for testing for CDI infections.
6. Diagnosis: It is essential for IPs, HEs, and the AS team to understand the scope of rapid diagnostic tests and work together to assist clinicians in interpreting and responding appropriately to results.
The second APIC paper published in 2019 was “Advancing the profession: An updated future-oriented competency model for professional development in infection prevention and control,” proposed the following actions for the IP role in ASP:5
1. providing consultative expertise
2. being a leader and advocate
3. identifying and detecting multidrug-resistant organisms
4. reporting surveillance trends over time
5. using surveillance data (e.g., treating asymptomatic bacteriuria, collecting contaminated specimens)
6. analyzing antibiograms and antibiotic use
7. assisting with early organism and infected patient identification
8. promoting compliance with standard and transmission-based precautions and other infection prevention strategies, such as care bundle practices and hand hygiene
9. developing and providing educational programs for staff, patients, and visitors
Other experts have recommended additional activities which arguably would cross the boundary into the competencies of other departments. For instance, participating in the production of the antibiogram as well as providing associated training, would seem to cross the boundary into responsibilities/competencies of the laboratory scientist.6,7 Also suggested to be within the purview of the IP is identifying bug-drug mismatches (i.e., whether a prescribed antibiotic is effective based on bacterial sensitivities). This would seem more within the purview of the pharmacist and physician.7-9
Not mentioned in any of these papers is arguably the most significant role for IPs in antibiotic stewardship programs – the prevention of healthcare-associated infections (HAIs). For every infection prevented, there are fewer antibiotics administered in addition to the associated resistance pressure, CDI risk, incidence, associated patient morbidity and healthcare cost. The APIC publications provide a high-level overview of the role of the IP in ASP, which can be built upon at the local level to provide more specific actions. Ongoing updates will be required moving forward due to the dynamic nature of the responsibilities of the IP.
Sue Barnes, RN, CIC, FAPIC is an independent clinical consultant, Board certified in Infection Control and Prevention, a Fellow of APIC (FAPIC) and co-founder of the National Corporate IP Director Network. She currently provides marketing and clinical consultation to select industry partners who seek to support infection prevention with innovative products.
References:
1. CDC Web page Antibiotic/Antimicrobial Resistance (AR/AMR) https://www.cdc.gov/drugresistance/
2. Dall C. New rule requires antibiotic stewardship programs in U.S. hospitals. Center for Infectious Disease Research and Policy; Sept. 26, 3019.
3. Weissenbach M. et al. Exploring the role of infection preventionists in antimicrobial stewardship programs through several lenses: A brief report. Am J Infect Control. 48 (2020) 106-107.
4. Manning, M et al. Antimicrobial stewardship and infection prevention—leveraging the synergy: A position paper update. A J Infect Control. Volume 46, Issue 4, 364 – 368.
5. Billings C et al. Advancing the profession: An updated future-oriented competency model for professional development in infection prevention and control. Am J Infect Control. 47 (2019) 602-614.
6. Moehring R et al. Challenges in Preparation of Cumulative Antibiogram Reports for Community Hospitals; J Clin Microbiol. Aug 2015, 53 (9) 2977-2982.
7. Perri L. The Infection Preventionist's Role in Antimicrobial Stewardship Programs. Infection Control Today. Oct. 6, 2017.
8. Al-Homaidan HT, Barrimah IE. Physicians' knowledge, expectations, and practice regarding antibiotic use in primary health care. Int J Health Sci (Qassim). 2018;12(3):18–24.
9. Duggan C, Joynes R, Rosado H. Pharmacy’s role in antimicrobial resistance and stewardship. Clinical Pharmacist. June 5, 2018.
Outbreak Readiness: How Prepared is Your Facility?
By Phenelle Segal, RN, CIC, FAPIC
Editor's note: This column originally appeared in the March 2020 issue of Healthcare Hygiene magazine.
For at least two decades, the U.S. has been planning for inevitable global pandemics, as evidenced by doubling of the National Institutes of Health (NIH) budget for biomedical research in 1998. The President’s Emergency Plan for AIDS Relief (PEPFAR) was created to stem the rising fear of devastation from Human Immunodeficiency Virus (HIV). However, health crises such as severe acute respiratory syndrome (SARS) that emerged in 2002, and Ebola in 2014, the U.S. response, together with the rest of the world, was considered slow and not well organized. Ebola proved that if basic systems had been in place, the epidemic could have been aborted at almost no cost, compared to the $5.4 billion that the U.S. funded.
Curbing epidemics is complex and requires a combination of money, additional manpower and with modern technology, the ability to diagnose, treat and prevent these diseases should be simpler.1
This article focuses on improvements nationwide for pandemic preparedness using Ebola’s arrival in the U.S. in 2014. Ebola Virus Disease (EVD) created an urgent need for pandemic preparation when the primary patient responsible for introducing the virus into the country fell through the cracks after his initial visit to a hospital in Texas. Ebola preparedness placed a heavy financial and human resource burden on healthcare facilities across the nation. Acute-care hospitals were provided guidance by the Centers for Disease Control and Prevention (CDC) via their “Interim Guidance for Preparing Frontline Healthcare Facilities for Patients Under Investigation (PUIs) for Ebola Virus Disease (EVD).” CDC guidance also included a detailed checklist for hospitals and specified that this could be used for Ebola as well as other infectious diseases. The result was much-improved awareness and preparedness for the inevitable; however, the question remains whether the healthcare industry can ever be fully prepared?
Novel respiratory viruses including severe acute respiratory syndrome (SARS Co-V) in 2003, H1N1 influenza (swine flu) in 2009 and Middle East respiratory syndrome (MERS Co-V) in 2012 reminded the world that ongoing preparation -- particularly in the acute-care setting -- is vital to the success of preventing an outbreak of major magnitude.
Once again, the U.S. currently faces the threat of a respiratory virus outbreak with the novel coronavirus known as COVID-19 that originates from and has sickened tens of thousands of people in China. The death toll has surpassed 1,500 at the time of writing. Similar to SARS and MERS, most often the virus spreads from respiratory droplets as a person-to-person transmission, when a person who is infected sneezes or coughs within the space of approximately 6 feet of others. As this novel virus has many unanswered questions to date, it is not certain whether surface contamination can infect mucus membranes including the mouth, nose or eyes.
Are We Prepared?
In October 2018, the U.S. Department of Health and Human Services (HHS), Office of Inspector General (OIG) released a report, “Hospitals Reported Improved Preparedness for Emerging Infectious Diseases After the Ebola Outbreak.” The OIG found that most acute-care hospitals in the nation were unprepared for the outbreak of Ebola in 2014, “…with 71 percent of hospital administrators reporting that their facilities were unprepared to receive Ebola patients. By 2017, administrators from only 14 percent of hospitals reported their facilities were still unprepared for emerging infectious disease (EID) threats such as Ebola.” Hospitals began updating their emergency plans, provided education and training for staff, particularly front-line staff, purchasing additional supplies and the very important task of conducting drills. HHS provided many resources, and these are available to date. The greatest challenges for hospitals to maintain preparedness includes immediate and day-to-day priorities taking precedence, preparing for natural disasters and staff time. In December 2014 it was reported that state health officials had designated 35 hospitals as “Ebola centers” and were ready to accept patients if necessary.
Pandemic Preparation for COVID-19
Outbreak or pandemic readiness is multi-layered and requires effort at the federal, state, local and individual facility levels, as evidenced by Ebola.
Pandemic preparation guidance for COVID-19 is changing daily as the experts learn more about this evolving illness. CDC continues to provide ongoing updates to healthcare professionals. These guidelines are extensive, and many resources are available for healthcare professionals in acute-care hospitals and for emergency medical service (EMS) personnel. Guidance for outpatient care and other inpatient facilities has not been provided at this juncture; however, the CDC does recommend that all healthcare providers and facilities refer to the guidelines to keep updated on the evolving situation. Key components to effective containment of this emerging virus include the following:
Evaluating and Reporting Persons Under Investigation (PUI)
The CDC clinical criteria for a 2019-nCoV person under investigation (PUI) have been developed based on what is known about MERS-CoV and SARS-CoV and are subject to change as additional information becomes available. Healthcare providers should obtain a detailed travel history for patients being evaluated with fever and acute respiratory illness. The CDC’s guidance for evaluating and reporting a PUI for MERS-CoV remains unchanged.
Criteria to Guide Evaluation of Persons Under Investigation (PUI) for 2019-nCoV
For any patient meeting criteria for evaluation for COVID-19, clinicians are encouraged to contact and collaborate with their state or local health department. For patients that are severely ill, evaluation for COVID-19 may be considered even if a known source of exposure has not been identified
Recommendations for Reporting, Testing and Specimen Collection
Healthcare providers should immediately notify both infection control personnel at their healthcare facility and their local or state health department in the event of a PUI for 2019-nCoV. State health departments that have identified a PUI should immediately contact CDC’s Emergency Operations Center (EOC).
Interim Healthcare Infection Prevention and Control Recommendations for Persons Under Investigation for 2019-nCoV
This section of the guidance is extensive and includes but is not limited to “Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings.
PPE for Healthcare Personnel
As the guidance states, “Healthcare personnel can protect themselves when caring for patients by adhering to infection prevention and control practices, which includes the appropriate use of engineering controls, administrative controls, and personal protective equipment (PPE). The CDC has issued guidance recommending the use of PPE for healthcare personnel caring for patients with confirmed or possible 2019-nCoV infection.”
In summary, emerging pathogens capable of spreading easily from person to person create a vulnerable and potentially dangerous situation worldwide, with the threat of outbreaks at any time. Immunity is usually absent, resulting in potentially severe repercussions for infected patients. History has shown that four influenza pandemics have occurred between 1918 and 2009. In addition, Ebola, the first hemorrhagic viral disease arrived in 2014.
Government, state and local agencies are working diligently to ensure that guidelines and resources are available for healthcare professionals, including those working in acute-care facilities, to prepare for an isolated patient or an influx of patients. It is the responsibility of these facilities to ensure that action plans for pandemic preparedness are developed, implemented, enforced and tested by performing drills at various times, to ensure that at any moment in time, they are prepared for the inevitable.
Phenelle Segal, RN, CIC, FAPIC, is president of Infection Control Consulting Services.

What to Look for in a Vendor Partner
By Linda Homan, RN, BSN, CIC
This article originally appeared in the February 2020 issue of Healthcare Hygiene magazine.
When it comes to infection prevention, we all want a silver bullet, a quick fix that cuts through complexity and provides an immediate solution to a problem. In truth, there is no silver bullet, but there are fundamental infection prevention measures that are proven to be effective in reducing healthcare-associated infections, such as hand hygiene, environmental hygiene, and instrument reprocessing.
In 2010, Wenzel and Edmond introduced the concept of horizontal and vertical infection prevention measures.1 Vertical measures are pathogen-based, reducing infection or colonization caused by specific pathogens in selected patient populations. They are often higher cost interventions as they may involve a microbiologic screening test, and they often are more resource intensive. Examples of vertical interventions are nasal decolonization to prevent transmission of MRSA, MDRO active surveillance and isolation precautions all of which are labor intensive and add cost to patient care. Horizontal measures are already part of routine patient care, are applied to all patients and are equally effective against superbugs as they work against garden-variety organisms.
Horizontal measures are generally less costly than vertical interventions and are consistent with patients’ need to avoid all infections, not just those due to specific organisms.2 The challenge is that horizontal measures often require modification of the day-to-day behaviors of healthcare workers, which means they are more difficult to sustain. They require ongoing education and feedback around a standardized process and buy-in from healthcare workers themselves in order to consistently practice the desired behavior. Hand hygiene, environmental cleaning and disinfection, and instrument reprocessing are horizontal measures and they include not only efficacious products, but also evidence-based processes and diligent practice by healthcare workers.
Product + Process + Practice = Sustained Performance Improvement
When it comes to horizontal infection prevention measures, it is not enough for vendors to offer a product and make a sale. Vendors should be held to a higher standard – they should be vendor partners. A vendor partner is an extension of your team and an asset to your hospital’s success. They are a partner who works with you from identifying a need and supplying a solution, to implementing and sustaining improvement with your facility’s team. It is not transactional, and it is not just a product.
Why should hospitals expect this level of service from their vendors? Because healthcare is complicated and changing quickly. Hospital margins are being pinched. Staff are being asked to do more with less. There are emerging pathogens that are threatening patient safety. A vendor partner’s goal should be your ongoing success as a healthcare provider – sustained performance improvement. But, in order to succeed in this new healthcare environment, we must move past that transactional relationship to a partner relationship that holds vendors to a higher standard and makes them part of a holistic, long-term solution.
A good vendor partner will provide:
- A strong business case to help stakeholders understand the value of the solution
- Data and actionable insights that are easy to understand and drive continuous improvement
- Education in a variety of formats and languages
- Timely and comprehensive on-site service
- A solution that easily integrates into existing workflows
- Onsite customer support to ensure a solution’s success
They must also be willing to partner with customers to standardize processes and improve healthcare worker practices. This requires evidence-based protocols, education and objective performance feedback so that hospitals understand exactly how they can make improvements. It’s a partnership that addresses not only at the product, but the processes and practices that will deliver performance.
I have worked on the business side of infection prevention for many years, but prior to that I was a practicing infection preventionist, certified wound care specialist and nurse manager for just as many years. In the infection prevention and wound specialist roles I worked with many vendors. Some were transactional – they would try to sell me something and, once sold, walk away - no educational support, no follow up, no ongoing connection. Others were more focused on establishing trust and partnership.
One of the most influential people in my career in those days was a sales representative for a wound care company. She was a wound, ostomy continence nurse herself prior to going into sales, and she taught me a lot -- not just about the dressings she was selling, but about wound care itself. She encouraged me to gain expertise that enabled me to take the exam to become a certified wound care specialist, which I did. I went on to become an infection preventionist and, recognizing the value of certification, quickly became certified in infection prevention and control.
The point of this short autobiographical sketch is to highlight the different approaches that manufacturers and their representatives have toward the customer. Some are transactional, some are partners. My wound care sales representative wasn’t just selling me a product, she was a consultant, providing me with the tools and information I needed to help my team improve patient care. Depending on what you are purchasing, either approach might be right. If you are purchasing tongue depressors, a transactional approach makes sense. However, if you are purchasing something more complex that needs to fit into your facility’s workflow, such as a product or service that has an impact on patient outcomes and hospital margins, then a vendor partnership is in order because they will help you see blind spots and opportunities for improvement and help you incorporate them into your facility’s operations. It is another set of eyes, a helping hand, a partnership. It makes sense.
Here are some things to look for in a vendor partner throughout the sales cycle:
Pre-sale
Before vendor partners suggest a solution, they should ask you about your facility. They should be listening to you and your challenges – problems that you’re trying to solve but haven’t been able to yet. Once they understand your operations, only then can they suggest solutions that can meet your needs. They should also be asking you about your facility’s demographics such as:
O Basic facility statistics (size, beds, etc.)
O Facility ratings
O Publicly available infection rates
O Patient population in your hospital
O Hospital and system strategic initiatives
Before asking for your business, vendor partners should provide you with strong, evidence-based resources to support their products. You don’t need to see clinical studies to decide which tongue depressor to buy, but, if the vendor is claiming to improve patient outcomes or operational efficiency, evidence is needed. Here is an example: not all efficacy claims need to be supported by a randomized, controlled trial. If a disinfectant has an EPA claim as a sporicide, a clinical study to prove that it kills spores is not necessary because EPA registration ensures that the product kills spores. The claim that the same sporicide reduces C. difficile infection rates when used as part of an overall environmental hygiene program, however, should be substantiated by clinical evidence.
Vendor partners should also be sharing their knowledge of industry trends. They focus on emerging issues and technology in their area of expertise and can help you “see around the corner” so that you’re prepared for what is coming next.
Some products and services require a trial before deciding to purchase. While your hospital may have unique circumstances to take into consideration, a strong vendor partner has a well-defined process to ensure an effective trial that can account for your circumstances. They will also have the resources (product, tools, training and people) to support the trial so that you aren’t taking on all the work by yourself. Remember, their job is to make your work better.
Before a trial begins though, it is critical that you and the vendor partner agree on metrics for success. The success criteria should be objective, measurable and achievable within the timeframe of the trial. For instance, while a product may help reduce healthcare-associated infections, the outcome measure of infection rate reduction is not measurable within the timeframe of a one-month trial. Rather, evaluate the process measure. During a trial for an electronic hand hygiene compliance monitoring system, one can measure the impact of the system on the process measure of hand hygiene compliance, but not the outcome measure of HAI reduction – that simply requires more time.
Value Analysis
Vendor partners help provide you with talking points for key conversations with hospital stakeholders by anticipating what questions will be asked, knowing stakeholder priorities, and providing appropriate data to share.
Once you’ve decided to take a product to the value analysis committee, vendor partners can help you prepare messaging that presents your case convincingly and helps stakeholders understand why they need to take your recommended actions. They do this by helping you:
O Target your message to the audience. Top priorities for a c-level executive are different than those of clinical staff, for instance, and top priorities for a CEO are not the same for CFOs or COOs either.
O Make strong comparisons. Compare the value of the solution you are recommending to what is currently being done.
O Bring the evidence. Provide well-supported research, studies, and other data that support your recommendation and resonate with your stakeholders.
Implementation
The collaborative vendor partner’s work is just beginning once the product has been approved for purchase. Work with your vendor partner to map out the implementation timeline and process. They should provide in-person education and training along with leave behind train-the-trainer resources for you to use when training new employees or providing refresher training.
And, because it’s difficult to measure or make improvements without good data, digital technology plays an increasing role in this space because it provides hospitals with actionable insights that they can use for continuous improvement. Vendor partners should provide comprehensive training on the collection, analysis and reporting of any insights that are derived as part of the product or service.
They will also help you evaluate what’s working and make contingency plans for addressing results that aren’t what you expect.
Ongoing support and partnership
Ongoing support and partnership are key deliverables from a vendor partner. The relationship doesn’t end with a purchase. Vendor partners should review data, provide education, follow up and service on a regular, mutually agreed upon cadence to ensure that you’re reaping the benefits of said solution. This is especially important when the solution being implemented is intended to drive behavior change such as hand hygiene or environmental hygiene compliance – it simply doesn’t happen overnight. It is a process that is optimized over time to accommodate your facility’s evolving needs.
Conclusion
When solving for complex issues that require behavior change, hospitals should be looking beyond products for a more holistic and long-term solution. Hospitals can improve results by partnering with vendors who work alongside them to develop lasting, customized, and programmatic solutions that address their specific needs. Something I think hospitals expect, but shouldn’t, is that improvements will fade (regress to the mean) over time. In fact, they should expect and be armed with the products, processes and practices that will continuously improve their performance over the lifetime of the solution.
Change can be hard, especially when it involves adjustments to behavior, but with the right vendor partner it is possible for hospitals to make comprehensive and sustainable improvements to horizontal measures that impact clinical and operational outcomes, while also cultivating the financial wellness of the hospital. Products alone simply don’t cut it anymore – hospitals can and should expect more from their vendor partners.
Linda Homan, RN, BSN, CIC, is senior manager of clinical affairs for Ecolab Healthcare.
References:
- Wenzel RP, Edmond MB. Infection Control: The case for horizontal rather than vertical interventional programs. Int J Inf Dis 2010; S3-S5.
- Edmond MB, Wenzel RP. Screening Inpatients for MRSA — Case Closed. N Engl J Med 2013; 368:2314-2315.
The Role of the Infection Preventionist in Product Purchasing
By Sue Barnes, RN, CIC, FAPIC
This column originally appeared in the February 2020 issue of Healthcare Hygiene magazine.
As healthcare costs continue to rise, the process of selection of clinical products must be objective and scientific. Because there are so many elements involved during this process, coordination by the value analysis committee is critical to ensuring both patient safety and cost containment. In the role as a core member of this committee, the infection preventionist (IP) serves a number of functions including:1,2
• Bringing formal proposals for the introduction of infection prevention products/technology incorporating evidence of efficacy and estimated return on investment (ROI);
• Providing consultation regarding the safety and efficacy of less expensive products supporting prevention of HAI, that may be proposed by the committee as a cost saving measure;
• Providing important guidance to ensure that any product or technology introduced can be effectively cleaned and disinfected if used on or around patients, and to ensure that the recommended products for cleaning/disinfecting are compatible with those in use at the facility;
• Supporting the committee’s assurance of a vendor’s capacity to provide adequate staff training in real time so that the product/technology will be used appropriately and result in optimal outcomes;
• Ensuring that any infection prevention product meets all evidence-based clinical guidelines and recommendations from regulatory and clinical organizations including the CDC.
Related to and supporting these functions are the additional important roles played by effective IPs, of early adopter and principle investigator for trials of innovative products supporting prevention of healthcare associated infection (HAI).3,4 A classic example of the IP role as early adopter has been demonstrated with the range of chlorhexidine gluconate (CHG) containing products. It was far in advance of randomized clinical trials proving efficacy of CHG in reducing infection risk, that IP departments began championing CHG based products starting with healthcare hand soap in the 1970s.5 It subsequently became a community standard and then decades later the Centers for Disease Control and Prevention (CDC) finally added it as a recommended practice in 2002.5 Similar time gaps can be seen between implementation of many other CHG containing products and the publication of randomized controlled trials and clinical guideline integration for infection prevention, including vascular access skin prep, impregnated central venous catheters, impregnated surgical and vascular dressings.5 In the absence of patient risk, many IPs champion products based on early evidence of efficacy in order to optimize patient safety. It is a certainty that many patient lives have been saved as a result of this philosophy of early adoption.
The role of principle investigator and/or participant in studies of innovative products is equally important in the quest for zero preventable HAI. From simple before and after studies, to large double blind randomized controlled studies, IPs have participated in and led trials of innovative products designed to reduce HAI risk, building the evidence base for efficacy. This typically initially leads, often only after many years. to establishing a community standard, and then much later to inclusion in clinical guideline(s).6
IP and Industry Collaboration
From the frontlines of healthcare in hospitals and clinics to the corporate offices of the Association for
Professionals in Infection Control and Epidemiology (APIC), IPs work collaboratively with industry partners to
introduce innovative products and technology designed to optimize patient safety by reducing HAI risk.7 At the
corporate level the APIC Strategic Partner Program is a formal, mutually beneficial partnership between APIC
and Industry Partners united in the common goal of reducing the risk of infection. The industry partners play an important role in supporting many of the programs and services that makes the APIC membership so valuable. More recently Industry Perspectives has been introduced by APIC, an online resource for IPs and healthcare workers to stay up-to-date on products, services, research, and innovation relevant to the field of infection prevention and control.
An important opportunity for IP professionals at all levels to learn about new infection prevention products, and develop relationships with industry partners, occurs annually during conferences including the annual meetings of APIC and the Society for Healthcare Epidemiology of America (SHEA). When visiting the vendor exhibit-hall during these conferences it’s helpful to be prepared with a few standard questions for vendors such as:
1. What studies providing evidence of efficacy have been published in peer reviewed journals and/or presented at conferences?
2. Does the data available address reduction of bacterial loads only, or also reduction of infection rates?
3. Can the vendor connect you with an IP at another facility using the product with good results?
Industry partners often offer a range of supportive services that can be leveraged by IP departments to reduce diversion of constrained IP resources. For instance, since tracking of compliance with appropriate product use is time consuming, this is a significant value-added service often provided by industry partners. Most vendors are also willing and able to partner with the clinical teams to provide direct observation, coaching and teaching when new product(s) are introduced. Collaboration between industry partners and IP professionals simply makes patients safer.
References:
1. Henry A. Product Evaluation. APIC Text Online Chapter 5; October 3, 2014.
2. Valenti W. Infection control and product evaluation. Infectious Disease Advisor - Hospital Infection Control. 2017.
3. Conway L et al. Tensions inherent in the evolving role of the infection preventionist. Am J Infect Control. Vol. 41, No. 11, 959-964.
4. Barnes S, et al The emerging role of the corporate or system-level infection prevention director for integrated delivery networks. Am J Infect Control. Vol. 47, No. 6, 638-642.
5. Chlorhexidine Facts: https://chlorhexidinefacts.com/
6. Pyrek K. Injecting the research and resources into infection prevention. Infection Control Today. May 17, 2018.
7. Humphreys H. New technologies in the prevention and control of healthcare-associated infection, J R Coll Physicians Edinb. 2010 Jun;40(2):161-4.
Leading the Way to Zero: Moving Purposefully Forward Together
By Sylvia Garcia, MBA, RN, CIC
This column originally appeared in the January 2020 issue of Healthcare Hygiene magazine.
At the opening of the 2006 annual meeting of the Association for Professionals in Infection Control and Epidemiology (APIC), then-APIC president Kathleen Arias said, “Zero tolerance is not a number—it’s a culture in which healthcare providers strive to prevent as many healthcare-associated infections as possible. We may never eliminate every infection, and many cannot be prevented, but infection control professionals should accept nothing less than the very lowest rates of infection.”
Back then, I sat in the audience and thought to myself, great idea, but is it achievable? Which infections should we prioritize? What are the key interventions? How do we get support from leadership and staff? (I wasn’t even thinking about the patient or their family at that point.)
There were already evidence-based guidelines available from Centers for Disease Control and Prevention (CDC) and other professional organizations on a variety of key topics. The next year, the Centers for Medicare & Medicaid Services (CMS) published payment reforms intended to increase emphasis on value-based purchasing which identified central line-associated bloodstream infections (CLABSI) and indwelling catheter-associated urinary tract infections (CAUTI) as “never events.” So, I knew CLABSI and CAUTI would be on leaderships’ list of priorities, but was this enough?
The answer would become clearer during 2008 when the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), the American Hospital Association (AHA), APIC, and the Joint Commission worked together to create the Compendium of strategies to prevent healthcare-associated infections in acute-care hospitals. These documents focused on implementation of basic strategies to prevent the most common healthcare associated infections (HAIs) as well as providing special approaches when basic practices were not enough. They also recommended that accountability be assigned and proposed performance metrics to monitor quality improvement efforts.
Information from the CDC, the Compendium and other professional organizations soon became an even greater organizational priority when the Joint Commission added three new requirements to national patient safety goal (NPSG) 7: Reduce the Risk of Healthcare Associated Infection in 2009 and an additional topic area in 2012
• Implement evidence-based practices to prevent health care–associated infections due to multidrug-resistant organisms (MDRO)
• Implement evidence-based practices to prevent CLABSI
• Implement evidence-based practices for preventing surgical site infections (SSI)
• Implement evidence-based practices to prevent CA-UTI
Today, the results of concentrated efforts to identify key interventions and reduce risk by implementing evidence-based practices are clear. Nationally, among acute care hospitals, significant progress has been made. For example, between 2017 and 2018, an 8 percent to 12 percent statistically significant decrease in CAUTI, CLABSI and hospital-onset C. difficile infections was reported. However, there was no significant decrease in SSI rates.
According to point prevalence surveys of hospitals conducted in 2011 and then again in 2015, there has also been a statically significant (p<0.0001) decrease in HAI amongst hospitalized patients: 1 in 25 (4 percent) versus 1 in 31 (3.2 percent), respectively. Pneumonia, gastrointestinal infections (most of which were due to Clostridium difficile) and surgical-site infections were the most common health care-associated infections infection identified.
As the following NPSGs are moved to standards effective July 1, 2020, organizations need to continue to implement evidence-based practices.
• NPSG.07.03.01—Multidrug-resistant organisms
• NPSG.07.04.01—Central line–associated bloodstream infections
• NPSG.07.05.01—Surgical site infections
• NPSG.07.06.01—Catheter-associated urinary tract infections
Organizations should also be aware that in November 2019, the CDC released a report about the threat of antibiotic-resistant organisms and the statistics are eye-opening: “…antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and 35,000 deaths in the United States each year. That means, on average, someone in the United States gets an antibiotic-resistant infection every 11 seconds and every 15 minutes someone dies.”
To keep patients, visitors and staff safe, organizations should be ready to implement CDCs recommended containment strategies when these organisms are identified. This includes ensuring compliance with existing Joint Commission focus areas, including:
• Implementation of standard and transmission-based precautions
• Making appropriate personal protective equipment available to staff
• Training staff on selection, limitations, maintenance, donning and removal of personal protective equipment
• Enforcing use of appropriate personal protective equipment
Note: Examples of potential survey findings related to the aforementioned areas were published in the August 2019 edition of Perspectives, under the “Consistent Interpretations” section.
We are making progress but there is still much work to be done both for the common infections that occur in healthcare such as SSI, and those, such as antibiotic resistant organism and other high- consequence organisms, that loom on the horizon.
Each healthcare organization needs to look within and conduct an accurate risk assessment – and ask: where are the low hanging fruit and the biggest risks? Are leadership, staff, patients, their families and their significant others are involved? And, is everyone working together to prioritize, plan, implement, and monitor?
If we all hold ourselves and our colleagues responsible and accountable…together we can get to zero HAIs!
So, 14 years later, do I think that we can achieve zero HAIs? My answer is a resounding Yes!
Sylvia Garcia, MBA, RN, CIC, is director of infection prevention and control within the of Division of Healthcare Improvement at the Joint Commission.
References:
1. Association for Professionals in Infection Control and Epidemiology. Prevention Strategist. 40 Years of Growth and Progress. Winter 2012.
2. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program: changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Federal Register. 2007;72(162):47129–48175.
3. Centers for Disease Control and Prevention. 2018 National and State Healthcare-Associated Infections Progress Report. Available at: https://www.cdc.gov/hai/data/portal/progress-report.html
4. Magill SS, et.al. Changes in prevalence of healthcare associated infections in U.S. Hospitals. N Eng J Med. 2018 Nov 1;379(18):1732-1744. doi: 10.1056/NEJMoa1801550
5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States – 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf .
6. Centers for Disease Control and Prevention. Containment Strategy Responding to Emerging AR Threats. Available at: https://www.cdc.gov/hai/containment/index.html.
Screening for Asymptomatic Bacteriuria: A Dangerous Intersection
By Barbara DeBaun, MSN, RN, CIC
This column originally appeared in the December 2019 issue of Healthcare Hygiene magazine.
It was a dark and stormy specimen. You know the story. It begins with a well-meaning nurse who notices that the urine in the patient’s urinary drainage bag is dark in color. When the urine is drained from the bag, the nurse notes that the urine is not only concentrated but smelly. More likely than not, this nurse will collect a sample of the urine and request an order for urinalysis and culture. The nurse has seen this before and is confident the patient’s symptoms suggest a urinary tract infection.
Another twist on the story is the patient who presents in the emergency department (ED) and whose daughter or son insists that “When mom gets like this, it’s always a urinary tract infection.” Sound familiar?
Asymptomatic bacteriuria (ASB) is the presence of one or more species of bacteria growing in the urine at specified quantitative counts (≥10⁵ colony forming units (CFU/mL or ≥10⁸ CFU/L regardless of whether there is presence of pyuria or signs/symptoms that are attributable to an urinary tract infection.
What do we know about ASB?
- Present in >30 percent of nursing home patients and 100 percent of those who are chronically catheterized
- 23 percent to 50 percent of antibiotic days for UTI are unnecessary treatment of ASB
- ASB is a benign condition that generally does not require treatment
Urine culturing misadventures often begin when a patient with a low pre-test probability of having a UTI is tested for one. It may start when a physician orders a urinalysis and culture on a patient who is unlikely to have a UTI, or in the scenario previously described, when a nurse obtains the specimen first and requests the order later. The integrity of the specimen including technique for obtaining and transporting it will impact the result.
Despite our best efforts, we still hear of urine samples being obtained directly from urinary catheter drainage bags. It is not unusual for a urine sample to be considered low priority for transfer to the lab therefore overgrowth of bacteria may result. The downstream impact of this includes additional work for the laboratory, increased costs for the pharmacy, and a negative impact on antimicrobial stewardship. Infection Preventionists are tasked with reporting hospital onset catheter associated urinary tract infections (CAUTI) and are likely reporting cases that are not true infections despite meeting the NHSN case definition. Financial penalties and impact on reimbursement are impacted by a substandard culture of culturing. The ultimate negative impact of culturing patients for an infection that is probably not likely, is that patients receive antibiotics that are not necessary.
The Infectious Diseases Society of America (IDSA) recently issued a clinical practice guideline for the management of asymptomatic bacteriuria. These 2005 guidelines recommended that only pregnant women and those scheduled to have an invasive urologic procedure be screened for ASB. The updated guidelines provide additional guidance on children and specific adult populations such as those with neutropenia, solid organ transplants, and surgery that does not involve the urological tract. Much has been learned about the impact of testing for ASB in these settings therefore the Society has provided guidance that will ultimately impact antimicrobial prescribing and the emergence of antimicrobial resistance.
An all-too-common practice is for practitioners to test a patient who has been admitted to an acute-care hospital with an indwelling catheter. The temptation to screen may be based upon the pressure to “capture on admission” or prove that the patient was already “infected” at the time of admission. The IDSA strongly advises against screening or treating ASB. As the screening of patients admitted with a catheter are likely to present in the ED, it is critical to partner with the ED providers and nurses so they are aware of the negative impact of performing urine screening in patients who are unlikely to have a UTI.
An additional strong recommendation is to avoid screening patients who are scheduled to undergo elective nonurological surgery for ASB. This is an area where the IP has tremendous opportunity to impact and drive change. Pre-operative order sets commonly include “urinalysis” and it may be “because we have always done it and we’re afraid to stop doing it.” We must have critical conversations with our surgical partners to discuss the impact of ASB screening to assure them that the risk of testing may outweigh the benefits. A patient scheduled for a knee replacement will be far better off if s/he is not treated with an antibiotic for ASB. There are no data to support the benefit of urine screening for nonurological surgical patients, however there is an abundance of data to connect antimicrobial therapy with negative downstream effects such as multi-resistant organisms and C. difficile infection.
Our laboratory “culture of culturing” practices that discourage the screening of patients who have a low probability of having a UTI directly impact antimicrobial prescribing practices and patient outcomes. This requires a partnership that connects the dots between laboratory stewardship and antimicrobial stewardship so that antibiotics are only prescribed when they should be.
Barbara DeBaun, MSN, RN, CIC, is an improvement advisor for Cynosure Health.
Education and Training of Frontline Infection Preventionists
By Matthew Hardwick, PhD
This column originally appeared in the November 2019 issue of Healthcare Hygiene magazine.
One of the most fundamental operations in any medical facility is cleaning. Cleaning critical areas such as patient rooms and surgical suites, as well as, less critical areas such as waiting rooms, hallways, cafeterias, kitchens, etc. are all apart of keeping medical facilities free of pests and, hopefully, pathogens. However, for the longest time, cleaning hospitals has followed the same basic principles as cleaning a hotel room -- clean visible soil. While there are cleaning protocols in place at nearly all medical facilities, the predominant feature of these protocols is routine cleaning with a focus on visible soil. It should come as a surprise to no one that pathogens do not always reside in visible soil. Indeed, many of the fluids and semi-solids that pathogens use to move from place to place are not readily visible to the naked eye (think sputum or fingerprint oils). Despite this fact, many cleaning protocols do not take invisible soil into account during both routine and specialized cleaning protocols. The responsibility for this lack of awareness belongs to all involved in healthcare. While environmental service (EVS) personnel, nurses and technicians are primarily responsible for keeping our medical facilities and equipment clean, they are only as good as they are trained to be.
From the Centers for Disease Control and Prevention (CDC) to the Association for the Healthcare Environment (AHE) to the Association for Professionals in Infection Control and Epidemiology (APIC), all agree that EVS personnel require extensive training to play their pivotal role in keeping healthcare environments free of pathogens. Indeed, each has dedicated significant resources to developing training programs for EVS workers. AHE has a suite of training programs aimed at frontline EVS workers to surgical suite cleaning to EVS management and leadership. Through funding from the CDC, APIC has developed training modules that run the gamut of cleaning from the basic principles, personal protective equipment, chemical safety, and techniques for cleaning and disinfection. In addition to these resources, vendors have developed their own extensive training for EVS workers. Such training is, perhaps, more individualized and often encompasses multiple days for training sessions and accompanied by annual refreshers for each worker.
- Fluorescent markers – This tool was developed in order to provide “before” and “after” feedback for EVS workers. In short, an invisible fluorescent gel is applied to pre-determined surfaces prior to EVS cleaning. Following cleaning, a manager uses a black light to determine if the gel has been removed from all the spots. In this way, the manager can determine adherence to cleaning protocols. It should be noted, however, that this method does not determine the efficacy of removing pathogens from environment.
- Adenosine Triphosphate (ATP) swabs – One way to determine if a surface is free of pathogens is to detect organic ATP (derived from biological organisms like bacteria, fungi and human cells) left on surfaces. ATP detection is performed only following cleaning, reducing the time needed for monitoring. In theory, this method will not only determine an EVS workers adherence to established cleaning protocols, it will also evaluate the protocol for pathogen removal. There is one big problem with this method, however. First, we do not know the half-life of biologically derived ATP, meaning that cells maybe dead (killed by auxiliary techniques such as UV and vapor) and still leave active ATP behind. In our laboratory, we have detected ATP signals in the absence of viable bacterial loads, more than 48 hours after exposure to UV light.
The development of fluorescent marker and ATP swab methodologies are a boon to the education and monitoring of EVS workers. However, given the limitations of both the current cleaning methodologies and these monitoring devices, we still have a long way to go before we have adequate tools to empower EVS workers.
While EVS personnel receive considerable training, nurses and technicians may only receive cursory training, if any at all, on how to clean medical equipment and how to use disinfectants. APIC’s training does include sections for healthcare professionals including a section on “roles and responsibilities” geared toward who cleans what in healthcare environments. Despite APIC’s efforts, there is clearly a gap in training for nurses and technicians. This education and training gap is critical, since these individuals are largely responsible for cleaning critical patient-care equipment such as blood pressure monitors and dialysis machines. Without understanding which disinfectant to use and the appropriate dwell times, as well as how to use wipers in order to reduce cross-contamination, we cannot begin to hope that healthcare surfaces will be cleaned properly.
In last month’s Healthcare Hygiene magazine, Linda Lybert and Caroline Etland described a comprehensive literature review commissioned by the Healthcare Surfaces Institute. As a part of this review, studies on current healthcare training and education were examined. Despite the availability of numerous training programs and studies to show that only 48 percent of healthcare surfaces are cleaned appropriately, no research studies were found to determine if these programs are effective. Rather, only a handful of research studies were focused on monitoring cleaning practices. This lack of scientific research into the effectiveness of EVS training is surprising and, frankly, appalling.
Given the rapidly evolving world of infection prevention, it is critical that all healthcare professionals – EVS workers, nurses, technicians – receive the education and training they need to fill their roles as frontline infection preventionists.
Matthew Hardwick, PhD is president/CEO of ResInnova Laboratories and is the president of the board of directors of the Healthcare Surfaces Institute. He is a thought leader in the field of infection prevention in the healthcare environment of care and is an expert in antimicrobial surface technologies.
When Prime Directives Collide: The Survival Wars
By Wava Truscott, PhD, MBA
This column originally appeared in the November 2019 issue of Healthcare Hygiene magazine.
The Prime Directive driving all humans is an internal instinct to survive. Throughout the ages, man has struggled and adapted to protect himself, his family, and his tribe. This core drive to survive has advanced weapons for protection, from crushing threats with rocks, to throwing spears, and on to more powerful weapons. To survive weather extremes, man huddled in caves, constructed lean-tos, and moved up to more permanent structures of wood, stone, and cement.
The same Prime Directive drives microorganisms to adapt or die. However, instead of innovative adaptations requiring thought, bacteria experience genetic mutations that may, or may not be helpful. Mutations that protect bacteria enable survival, while mutations that are not sufficiently protective die off with the bacteria. For example, those bacteria that received successfully mutations allowing them to withstand temperatures up to 180 degrees F, continue to thrive in the hot springs and steam vents of Yellowstone.
The adaptive capacity of bacteria has been incredibly successful, especially considering they have been on earth for over 1.8 billion years. Most successful mutations are passed on vertically from generation to generation in a long looped single chromosome composed of double-stranded DNA residing in the nucleolus of the bacteria. The instructional information is passed primarily during cell division, enabling future progeny to survive in their environment. These traits include such capabilities as biofilm formation for tribe protection, the ability of a small number of bacteria to form one-occupant spores, and the inherited capability that a few bacteria possess to produce small colony variant (SCV) progeny that, in effect, are invisible to the human immune system.
Some protective mutations can be transferred horizontally to other bacteria unrelated to the original “mutant.” The genetic instructions are encoded in a much smaller double-stranded DNA loop, the plasmid. Plasmids can replicate independently producing as many as needed to “share” with other bacteria. Once a bacterium receives a plasmid, it in turn produces replicates to fortify its own protection and potentially to distribute to others. Plasmids are also vertically passed on to progeny during cell division, thus improving the odds of survival for their descendants.
Antibiotic resistance genes are very successful mutations located on plasmids. The mutations only work on specific antibiotic types and only by specific action modes. There are at least 10 different protection modes that have been successful:
Within the bacterial cell itself:
- Blocks entry of specific antibiotic types trying to enter bacterial cell
- Flushes the antibiotic out of the bacterial cell before it reaches their targets
- Produces enzymes that break apart antibiotics before they reach their targets
- Produce antibiotic modifying enzymes that render the antibiotic ineffective
- Modify the targets so they cannot be impacted by the antibiotic
- Make so many clones of the antibiotic targets, that the antibiotic is spent before destroying all the targets
Within the group-protective biofilm:
- Exo-enzymes distributed throughout the biofilm matrix like land mines in the battlefield, digest specific antibiotic types when contact is made
- Many bacteria in the center of the biofilm, are altered into persister cells that shut down (hibernate), not allowing anything in---including antibiotics
- Most biofilm founding-bacteria attract diverse bacterial types to increase the odds of biofilm survival through genetically diverse protective adaptations
- Proximity and purpose of the (a) peripheral bacteria in a biofilm “fortress” makes them the forward perimeter guards. It is there that plasmids for diverse means of protection are most liberally shared. For example, the more means of defeating antibiotics each defender possess, the more effectively broad the antibiotic resistance. Bacteria deeper in the matrix are responsible for (b) harvesting moisture and nutrients, (c) metabolic waste disposal, (d) hibernating as persister cells, and (e) transforming into “Supper-Surface-Grippers.”
U.S. Daily Human Cost: Each day, approximately 5,000 Americans acquire a serious antibiotic–resistant infection. Of those, about 63 patients will die and a large percentage of survivors will suffer long term chronic consequences. By 2050, it is projected that untreatable antibiotic infections with overtake cancer as the number one cause of death globally.
U.S. Annual Financial Cost: Antibiotic-resistant infections add $20 billion in excess direct health care costs and up to $35 billion the additional costs to society for lost productivity.
As with any battle, attacking before the enemy can establish a foothold and fortify a reservoir of resistant pathogens is by far the easiest, most effective and least costly means of patient protection. It takes a team to do what’s needed including infection preventionists, OR and device reprocessing staff, environmental services, engineering, and clinicians.
Infection preventionists must have help to handle required reports, statistics and trending paperwork so they can be actively on the floors, teaching, advising, admonishing, finding solutions and supporting staff trying to do the right things under pressure.
Infection prevention efforts are becoming more and more imperative. We are facing a clash of Prime Directives between patient and pathogen. Bacteria have almost a 2 billion-year proven record of adapting to survive. We are losing the capability to treat more and more our patients’ infections. We need to adapt tactics, techniques, technologies and responsibilities if we are to win the war for patient survival.
Wava Truscott, PhD, MBA, is principal of Truscott MedSci Associates, LLC.
This article is from the October 2019 issue of Healthcare Hygiene magazine.
Legionella: Recognizing the Risk and the Resources
By Sylvia Garcia, MBA, RN, CIC
Every day, patients are at risk because healthcare facilities are not aware of hazards related to water systems and equipment that uses water, or they have not prioritized it as an important issue. It is estimated by the Centers for Disease Control and Prevention (CDC) that 9 out of 10 infections acquired in a healthcare setting could have been prevented if the facility had initiated a better water management system.
Shockingly, 1 in 4 patients who develop healthcare-associated Legionnaires’ disease will die, compared to one in 10 that will die from community-acquired pneumonia. Even more surprising is the fact that at least 80 percent of the Legionella cases that occur in healthcare facilities could have been prevented by implementing an effective water management program.
The most common sources of Legionella cases are showers, cooling towers, decorative fountains, and hot tubs but anything that can create droplets or aerosols could become a source. For example, putting tap water into a room humidifier could lead to infection. About half of Legionella outbreaks are linked to incidences associated with human error, such as a health care professional not following instructions for use of equipment.
Although Legionella is highly publicized, it is not the only risk related to health care water systems. At the Association for Professionals in Infection Control and Epidemiology (APIC) 2019 Conference, researchers reported that 22 percent of consultations conducted by the Division of Healthcare Quality Promotion (DHQP) were water related. Causes of patient infections were identified as preventable, had the healthcare organization properly utilized available information and followed procedures communicating the need for the organization to implement an effective water management plan. For example, use of consumer-grade humidifiers in an operating room was linked to an outbreak of nontuberculous mycobacteria. Yet, the 2003 CDC Guidelines for Environmental Infection Control in Health Care Facilities clearly state that this this type of humidifier has been linked to Legionella outbreaks.
As with other infection prevention and control challenges, organizations need to follow a standardized approach to reducing risk related to waterborne disease.
1. Regulatory Requirements. Organizations should know their state’s regulatory requirements. Sources include health department and building code requirement documents. New York has enacted state regulations that require hospitals and residential healthcare facilities to perform environmental assessments, implement sampling and management plans to sample their potable water systems for Legionella and institute control measures in the event of a Legionella exceedance. New York also requires cooling towers to be registered and monitored for Legionella. All states provide or employ Healthcare Associated Infection Liaisons to direct healthcare workers to relevant information.
It is also important to understand state reporting requirements for Legionella and to identify known or suspected outbreaks caused by waterborne pathogens. To meet these requirements, facilities must implement a system to identify and evaluate possible cases. A laboratory finding is usually the first step in identifying a possible case. However, the infection preventionist or other knowledgeable person is also needed to apply generally-accepted case definitions or create a case definition in the outbreak setting.
State building codes vary, but many states have adopted a version of the Facilities Guideline Institute (FGI). Organizations can gain access to relevant building codes or, depending on the year, can access the information directly via FGI’s read-only access. For example, FGI 2014 and 2018 state “provisions based on a risk-assessment plan shall be included in the heated potable water system to limit the amount of Legionella bacteria and opportunistic waterborne pathogens.” For the same reason, unsealed, indoor decorative fountains are prohibited in these versions. FGI also provides excellent references - including CDC Guidelines for Environmental Infection Control in Health Care Facilities, American National Standards/American Society of Heating, Refrigerating and Air-Conditioning Engineers Standard 188: Legionellosis: Risk Management for Building Water Systems, American Society of Heating, Refrigerating and Air-Conditioning Engineers Guideline 12: Minimizing the Risk of Legionellosis Associated with Building Water Systems and the American Society of Plumbing Engineer’s Legionella Control in Health Care.
2. Centers for Medicare and Medicaid Requirements. In July 2018, CMS updated its requirement to reduce Legionella risk in health care facility water systems to prevent cases and outbreaks of Legionnaires’ disease. The updated requirement makes certain that Medicare-certified hospitals, critical-access hospitals and long-term care facilities develop, implement and monitor the effectiveness of water-management programs to protect patients, visitors and staff from exposure to waterborne pathogens, including Legionella pneumophila.
3. Manufacturer Instructions for Use (IFU). Equipment that uses or is connected to water has specific plumbing, filter and/or maintenance requirements. For example: air gaps may be required for plumbing installations; cooling tower instructions-for-use may specify inspection criteria and biocides to maintain biological control; and equipment may indicate use of sterile water or specific frequency for maintenance. In addition, some equipment may specifically state that it is not appropriate for healthcare settings. Careful reading and compliance with IFUs are essential to preventing outbreaks.
4. Evidence-based guidelines and national standards (EBG). CDC, the American Society of Heating, Refrigerating and Air-Conditioning Engineers and many other organizations have created excellent resources for preventing waterborne illness. Key resources from CDC include 2003 CDC Guidelines for Environmental Infection Control in Health Care Facilities Guidelines for Environmental Infection Control in Health care Facilities and the Centers for Disease Control and Prevention Tool kit, which outlines elements of an effective water management system with focus on health care facilities. EGB will include the following key elements:
• Establish a water management team. There is flexibility in qualifications of team members. However, it is important to seek individuals with backgrounds in the following when forming a health care facility team: facilities management, microbiology, infection prevention, risk management and occupational health.
• Describe the building’s current water system. Create a diagram that highlights water points of entry, distribution, storage and use. Most facilities display building drawings that include their plumbing system, so that is a great place to start.
• Identify where Legionella and other pathogens can grow. Facilities should identify at-risk systems and equipment with respect to their components, installation, configuration, use and condition, as well as vulnerability of persons served by these systems.
• Determine control measures and standards for monitoring them. Control measures must be developed for each risk point. Facilities must determine what is planned to be checked to ensure that their control measures are effective. Examples include but are not limited to: monitoring compliance with routine maintenance, water temperature, pH, chlorine levels and cultures. Note: Routine culture testing for Legionella and other pathogens is not required by CMS but may be required by state or local regulation.
• Establish interventions when clinical limits are not met. The expectation is that facilities establish a plan for remedy if a suspected health care-associated case of Legionella is identified or suspected or if control measures are not being met.
• Make sure the program is functioning as designed and is effective. Validate that all control measures have been implemented as designed and procedures have been established to confirm the water management program is effectively controlling water-related hazards.
• Document and communicate. Facilities’ water management programs should be documented. It is important to inform those at risk of the facility plan in place. If a problem occurs, it is required that the incident is reported to the health department.
5. Create a Facility Water-Management Plan. Using the steps, create a team and sort through water management requirements. The Joint Commission looks for evidence of compliance by using following key elements:
• Facility risk assessment to identify where Legionella and other opportunistic waterborne pathogens (e.g. pseudomonas, Acinetobacter, nontuberculous mycobacteria, and fungi) could grow and spread, and to evaluate programs to protect the health and safety of patients. Relevant standards should be recorded for facility water systems or equipment containing or using water.
• A water management program that considers input from the following publications: American Society of Heating, Refrigerating and Air-Conditioning Engineers 188 and the CDC Toolkit. Developing a Water Management program to reduce Legionella growth and spread in buildings: a practical guide to implementing industry standards.
• Testing protocols and acceptable ranges for control measures, with results of testing and corrective actions taken when control limits are not maintained
The Joint Commission surveyors may ask to review IFUs for equipment that uses or contains water. They also may ask about circumstances that could put a facility’s cooling towers or water system at risk.
Surveyors may also ask for a facility’s plan to mitigate risk, which should include identifying:
• System startups and shutdowns
• Areas of the facility that are closed or have low census
• Changes to municipal water treatment
• Water main breaks
• Construction or renovation
• Fluctuations in source water temperature
• Cooling tower maintenance
A systematic approach will ensure that key requirements and prevention strategies are not missed when preventing waterborne pathogens. There is not one solution to this challenge. In fact, water management needs to be uniquely tailored to each health care facility’s building, equipment, water and conditions. Implementing an organized approach, maintaining correct background information and utilizing key resources will help keep people safe from waterborne pathogens.
 Sylvia Garcia, MBA, RN, CIC, is the director of infection prevention and control in the Division of Healthcare Improvement. In this role, she is responsible for the oversight of infection prevention and control for The Joint Commission. She has more than 30 years of experience in infection control in both hospital and long term care settings, as well as eight years of clinical microbiology experience. Most recently, she served as the director of infection control at University of Chicago Medicine and was also an intermittent consultant for Joint Commission Resources for 10 years. Garcia has provided infection prevention and control consultation, assessment and education in a variety of healthcare settings including hospitals, health clinics, ambulatory surgery, and dialysis centers both domestically and internationally. Her specialty areas of interest include disinfection and sterilization, dialysis, infection prevention during renovation and construction, and control of Legionella. One of the highlights of her career has been training healthcare professionals in Saudi Arabia as infection preventionists. She served as a test writer and reviewer for the Certification Board of Infection Control and Epidemiology, and has also authored numerous articles and book chapters related to infection control including a chapter in the APIC Text and the Cleaning, Disinfection and Sterilization Chapter in The APIC/JCR Infection Prevention and Control Workbook, Third Edition. Garcia earned a degree in biochemistry and molecular biology from Northwestern University, a master’s of business administration from the Keller Graduate School of Management, and her nursing degree from Truman College.
Sylvia Garcia, MBA, RN, CIC, is the director of infection prevention and control in the Division of Healthcare Improvement. In this role, she is responsible for the oversight of infection prevention and control for The Joint Commission. She has more than 30 years of experience in infection control in both hospital and long term care settings, as well as eight years of clinical microbiology experience. Most recently, she served as the director of infection control at University of Chicago Medicine and was also an intermittent consultant for Joint Commission Resources for 10 years. Garcia has provided infection prevention and control consultation, assessment and education in a variety of healthcare settings including hospitals, health clinics, ambulatory surgery, and dialysis centers both domestically and internationally. Her specialty areas of interest include disinfection and sterilization, dialysis, infection prevention during renovation and construction, and control of Legionella. One of the highlights of her career has been training healthcare professionals in Saudi Arabia as infection preventionists. She served as a test writer and reviewer for the Certification Board of Infection Control and Epidemiology, and has also authored numerous articles and book chapters related to infection control including a chapter in the APIC Text and the Cleaning, Disinfection and Sterilization Chapter in The APIC/JCR Infection Prevention and Control Workbook, Third Edition. Garcia earned a degree in biochemistry and molecular biology from Northwestern University, a master’s of business administration from the Keller Graduate School of Management, and her nursing degree from Truman College.
