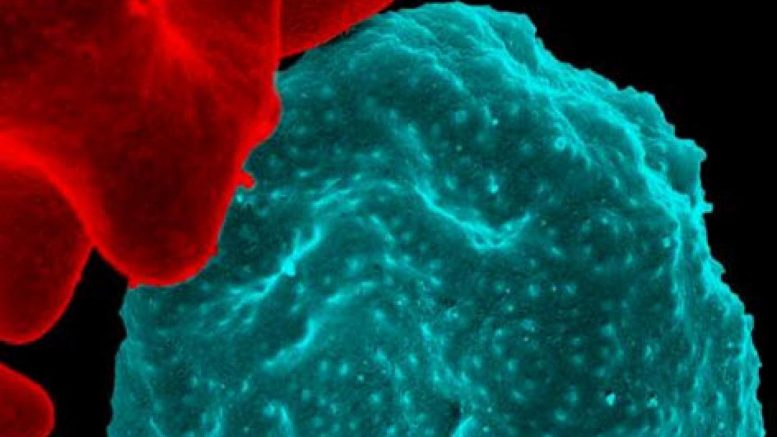
Colorized scanning electron micrograph of red blood cell infected with malaria parasites, which are colorized in blue. The infected cell is in the center of the image area. To the left are uninfected cells with a smooth red surface. Courtesy of NIAID
Researchers at the National Institutes of Health and other institutions have discovered another set of pore-like holes, or channels, traversing the membrane-bound sac that encloses the deadliest malaria parasite as it infects red blood cells. The channels enable the transport of lipids — fat-like molecules — between the blood cell and parasite, Plasmodium falciparum. The parasite draws lipids from the cell to sustain its growth and may also secrete other types of lipids to hijack cell functions to meet its needs.
The finding follows an earlier discovery of another set of channels through the membrane enabling the two-way flow of proteins and non-fatty nutrients between the parasite and red blood cells. Together, the discoveries raise the possibility of treatments that block the flow of nutrients to starve the parasite.
The research team was led by Joshua Zimmerberg, MD, PhD, a senior investigator in the Section on Integrative Biophysics at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study appears in Nature Communications.
In 2018, there were 228 million cases of malaria worldwide, leading to more than 400,000 deaths, 67% of which were among children under 5, according to the World Health Organization. In the current study, researchers determined that the channels through the sac, or vacuole, that encloses the parasite are made of Niemann-Pick C1-related protein (PfNCR1). The PfNCR1 channels are restricted to locations where the vacuole membrane touches the parasite’s membrane. The channels the team discovered in the previous study are formed by exported protein 2 (EXP2). Areas of the vacuole membrane containing EXP2 are located far from the parasite’s membrane, at an average distance of 20 to 40 nanometers. The researchers believe that the parasite may use this variation in distance to separate the two transport systems.
Reference: Garten et al. Contacting domains segregate a lipid transporter from a solute transporter in the malarial host-parasite interface. Nature Communications. 2020.
Source: National Institutes of Health (NIH)
Hi there! I could have sworn I’ve been to this blog before but after reading through some of the post I realized it’s new to me. Nonetheless, I’m definitely happy I found it and I’ll be bookmarking and checking back often!
Hi there, all the time i used to check weblog posts here early in the morning, since i love to gain knowledge of more and more.
Hi, just wanted to mention, I liked this article. It was inspiring. Keep on posting!
Thank you for any other informative web site. Where else may I am getting that kind of info written in such a perfect method? I have a challenge that I am simply now operating on, and I have been at the glance out for such information.
Having read this I thought it was extremely informative. I appreciate you finding the time and effort to put this article together. I once again find myself spending way too much time both reading and commenting. But so what, it was still worth it!
Thank you for some other fantastic article. Where else may anyone get that kind of information in such a perfect means of writing? I have a presentation next week, and I am at the look for such information.
What’s Taking place i’m new to this, I stumbled upon this I have found It positively helpful and it has helped me out loads. I hope to give a contribution & aid other users like its helped me. Good job.