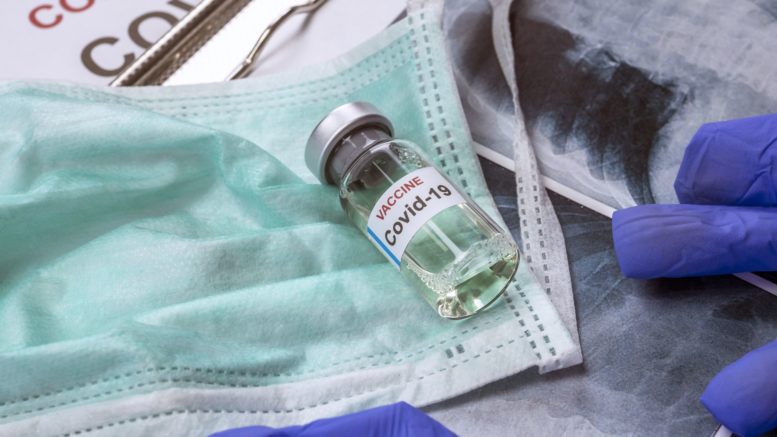
Under the leadership of President Trump, the U.S. Department of Health and Human Services (HHS) and Department of Defense (DoD) announced an agreement with Moderna, Inc. to manufacture and deliver 100 million doses of the company’s COVID-19 vaccine candidate. The federal government will own these vaccine doses.
Moderna will manufacture the vaccine doses while clinical trials are underway. Manufacturing in parallel with clinical trials expedites the traditional vaccine development timeline and builds toward the U.S. government’s Operation Warp Speed goal to begin delivering safe and effective vaccines to the American people by the end of the year. If the U.S. Food and Drug Administration (FDA) authorizes use as outlined in agency guidance, the vaccine doses would be distributed and used as part of a COVID-19 vaccination campaign.
“In creating a vaccine portfolio for Operation Warp Speed, the Trump Administration is increasing the likelihood that the United States will have at least one safe, effective vaccine by 2021,” said HHS secretary Alex Azar. “Today’s investment represents the next step in supporting this vaccine candidate all the way from early development by Moderna and the National Institutes of Health, through clinical trials, and now large-scale manufacturing, with the potential to bring hundreds of millions of safe and effective doses to the American people.”
The Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Office of the Assistant Secretary for Preparedness and Response, collaborated with the DoD Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense and Army Contracting Command to provide up to approximately $1.5 billion to manufacture and deliver the vaccine doses to government-designated locations across the country. The government also can acquire up to an additional 400 million doses of the vaccine.
The project announced today includes fill-finish manufacturing in U.S.-based facilities. This fill-finish manufacturing step ensures vaccine doses are packaged and ready to ship immediately, subject to successful clinical trials and FDA authorization.
If these doses are used in a COVID-19 vaccination campaign, the vaccine would be available to the American people at no cost. As is customary with government-purchased vaccines, healthcare professionals could charge for the cost of administering the vaccine.
The vaccine, called mRNA-1273, has been co-developed by Moderna and scientists from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. NIAID has continued to support the vaccine’s development including nonclinical studies and clinical trials.
In addition, BARDA has supported phase 2/3 clinical trials, vaccine manufacturing scale up and other development activities for this vaccine. The Phase 3 clinical trial, which began July 27, is the first government-funded Phase 3 clinical trial for a COVID-19 vaccine in the United States.
Source: HHS
Great post.
Hi, i think that i saw you visited my website so i came to return the favor.I am trying to find things to improve my site!I suppose its ok to use some of your ideas!!
No matter if some one searches for his essential thing, thus he/she needs to be available that in detail, so that thing is maintained over here.
Great article! This is the type of information that are meant to be shared around the internet. Disgrace on the seek engines for not positioning this submit upper! Come on over and visit my site . Thank you =)
I’m not sure why but this web site is loading extremely slow for me. Is anyone else having this issue or is it a problem on my end? I’ll check back later and see if the problem still exists.
Heya i’m for the primary time here. I came across this board and I find It truly useful & it helped me out a lot. I am hoping to provide something back and help others like you helped me.
This post will help the internet users for building up new website or even a blog from start to end.
At this time I am going away to do my breakfast, after having my breakfast coming yet again to read further news.
Hello there I am so excited I found your website, I really found you by mistake, while I was searching on Askjeeve for something else, Regardless I am here now and would just like to say thank you for a fantastic post and a all round enjoyable blog (I also love the theme/design), I don’t have time to browse it all at the minute but I have book-marked it and also added in your RSS feeds, so when I have time I will be back to read much more, Please do keep up the superb job.
I wanted to thank you for this great read!! I certainly enjoyed every little bit of it. I’ve got you book-marked to check out new stuff you post