Respiratory syncytial virus (RSV) is a common respiratory virus which usually causes mild, cold-like symptoms during the respiratory virus season. However, especially among young infants under six months of age or people over 65, RSV infection might turn more severe. Health complications associated with RSV include bronchiolitis (inflammation of the small airways in the lung), pneumonia or even sepsis which can require prolonged hospital stays.
Immunization products to prevent RSV disease in infants and older adults have been authorized in the European Union since 2022. These include long-acting monoclonal antibodies are given to newborns during the winter season as well as vaccines for pregnant people to protect newborns during their first winter season after birth. [1] The European RSV season has returned to pre-pandemic seasonality, lasting from around October to April.
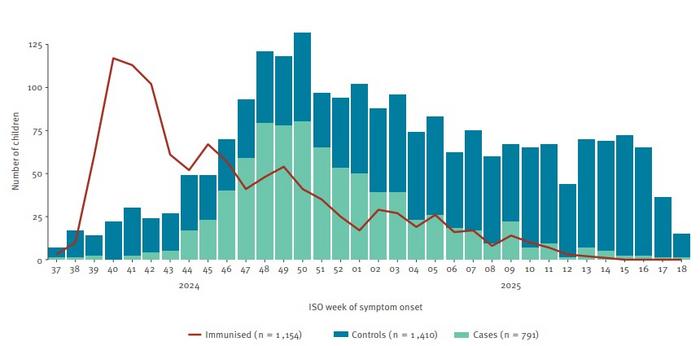
In their rapid communication published in Eurosurveillance, Savulescu, et al. looked at data from three European countries which had RSV immunization programs in place during the winter season 2024/25 to establish the effectiveness of the long-acting monoclonal antibody (nirsevimab) against RSV infection.
For their case-control study among children below the age of two years, the authors screened data from 4,102 hospitalized children in Belgium, Portugal and Spain between September 2024 and May 2025. Of those, 791 children who had been tested positive for RSV were included in the study analysis and 1,410 children who tested negative for RSV in the control group.
In the study, children were considered immunized if they received nirsevimab between September 2024 and May 2025 before testing, regardless of the dose, or their age and weight at the time of immunization.
Based on their analysis, Savulescu et al. conclude that “immunization of children after birth effectively prevented RSV-related hospitalization in children during the 2024/25 European winter season”. Among 2,201 children the pooled overall immunization effectiveness was at 79%, i.e. receiving long-acting monoclonal antibodies significantly reduced the risk of hospitalization due to RSV infection in this age group.
However, the level of protection declined over time following immunization: starting from 85% during the first month (<30 days) after immunization to 78% during days 30 to 89 after immunization and 69% after three months (90 to 215 days) from immunization.
Among infants aged 0–6 months, i.e. the newborns at highest risk for severe RSV illness, the overall effectiveness of immunization with long-acting monoclonal antibodies was 80%. The authors highlight that “effectiveness by time since immunization needs monitoring in future seasons.”
Source: European Centre for Disease Prevention and Control (ECDC)