New Guidance for Processing and Handling of Probes, Dilators and Accessories
By Susan Klacik, BS, CRCST, FCS, CHL, CIS, AAMIf
This article originally appeared in the June 2024 issue of Healthcare Hygiene magazine.
A new technical information report (TIR) from the Association for the Advancement of Medical Instrumentation (AAMI) offers guidance for the processing of ultrasound probes and dilators in health care facilities. The document, AAMI TIR99:2024 Processing of dilators, transesophageal and ultrasound probes in health care facilities, provides recommendation about the selection and use of cleaning, disinfection and sterilization systems that have been cleared by the U.S. Food and Drug Administration (FDA) to process the devices and their accessories. This article provides an abbreviated summary of the document’s content.
Cleaning and point-of-use treatment emphasized
Cleaning is among the most important steps in the device processing cycle, and the design and use of ultrasound devices present unique challenges to the cleaning process. Some transducers are designed and used in a manner that the handle and connector do not have direct patient contact (as opposed to the patient-contacting shaft and distal tip). Because of this, different methods are recommended for processing the patient-contacting and nonpatient-contacting components. Diligent adherence to the IFU is also essential to prevent device damage and infection.
Ultrasound transmission gel (USTG) and coupling agents, such as saline or lubricant, are critical for image quality. They are used with ultrasound-guided procedures and may be present on the device. During cleaning of contaminated dilators and ultrasound probes, personal protective equipment (PPE) should be worn. Further, policies and procedures should be developed and implemented to address all steps in the cleaning process, including point-of-use treatment, transporting from the point of use to the processing area, cleaning, rinsing and drying, and inspection.
A key difference with the processing of certain ultrasound probes is that the cleaning and disinfection process is performed at the point of use. This practice should be supported by a multidisciplinary risk assessment. TIR99 provides guidance on developing a policy to perform point-of-use treatment. This includes an algorithm for suggested workflow for transrectal, transvaginal and surface ultrasound device processing at the point of use. Recommendations are also provided for point-of-use treatment of the devices and their accessories to prevent soil from drying. The purpose is to reduce the level of bioburden and help prevent biofilm formation.
After point-of-use treatment is performed on probes and dilators, any devices not processed at the point of use are transported to the decontamination area. They should be transported in a collection system that meets Occupational Safety and Health Administration (OSHA) requirements (29 CFR 1910.1030) for transporting hazardous items. This includes labeling the devices with a biohazard label, placing them in a red bag, or other methods for distinguishing contaminated contents. When transporting contaminated probes used for a semicritical application, they should not have contact with the cable or plug, which does not undergo high-level disinfection. Separating the transducer from the cable and connector can be done by using a containment system with an impermeable divider or by placing the transducer inside a bag that then goes inside the rigid container. Before transport, probes should be prepared in a way that prevents organic soils from drying. It is possible to prevent drying by covering them with a water-moistened towel, applying a pretreatment product to only the area that can undergo immersion, or using a humidity chamber pack.
Once the probes and dilators are received in the decontamination room, the cleaning process begins. For transesophageal echocardiogram (TEE) transducers, a leak test should be performed before cleaning begins because immersing the probes in water with compromised integrity can damage the device. The leak test is to be performed according to the IFU, and the results of pass or fail should be documented. To prevent patient harm, TEE probes that have failed the leak test should be removed from service. Further, to prevent damage, TEE transducers should not be coiled too tightly.
TIR99 also includes recommendations for manual and mechanical cleaning. At the end of the cleaning process, the probes, dilators and accessory components should be visibly clean and inspected to verify that all visible soil was removed. The guidance document describes a different process for some components that do not come in direct contact with the patient. These components are addressed in the IFU and describe a manual cleaning process, followed by low-level disinfection (LLD) or intermediate-level disinfection (ILD). After a probe or dilator has been cleaned thoroughly, it then undergoes disinfection or sterilization. TIR99 provides guidance about the different types of disinfection and sterilization and how to ensure the processes are performed safely and effectively. Automated HLD systems are preferred over manual HLD processes because they are more reliable, reduce human errors and reduce the risk of personnel exposure to chemicals.
Drying, transport, storage and more
After HLD, the probe or dilator must be thoroughly dried to prevent waterborne pathogens, such as pseudomonas aeruginosa and other microorganisms, from surviving and thriving with resistant biofilm formation. All surfaces should be thoroughly dried with a clean, lint-free cloth or non-linting wipe before storage or use. Parts that are inaccessible or have lumens should be dried with pressure-regulated instrument air or HEPA-filtered air. A designated station or area to dry the probe or dilator is recommended.
HLD-processed probes or dilators should be visibly marked as “clean” or “patient ready” and then transported on a clean surface. Again, it is important to stress there is a certain way to transport probes with a cable or plug that has not undergone HLD, and those parts may be attached to a component that has undergone HLD. Again, the high-level disinfected probe portion of the equipment should be separated from the cable or plug by covering the probe with a visibly clean storage cover or using a containment system.
When storing the devices, avoid mixing contaminated items with cleaned, disinfected or sterilized items. Further, probes and dilators should be stored under environmentally controlled conditions, in a manner that protects them from contamination. TIR99 provides recommendations for storage in accordance with the Spaulding classification. An accepted maximum storage time for HLD-processed ultrasound probes and dilators to be considered safe for patient use is not provided in the guidance document. Therefore, it is recommended that a multidisciplinary team be developed (i.e., Sterile processing professionals, infection preventionists, risk management staff and other clinical personnel). The team should conduct a risk assessment to determine the maximum storage time for an ultrasound probe or dilator before it is no longer safe for patient use. ANSI/AAMI ST91:2021 provides a list of considerations that should be reviewed in the risk assessment.
Documentation is another critical step in medical device processing, including for probes and dilators. Such documentation can be paper-based or automated (digital). Documenting all steps in the process provides information that the cycle parameters have been met, while also elevating accountability. Most importantly, documentation can be used to trace a device to a source of an infection and determine whether a recall is necessary—and the extent of a recall. Note: Every healthcare facility should have a policy and procedure for product recalls. For improved traceability, probes and dilators that have undergone HLD or sterilization should have the documentation linked to the associated patient record.
Quality assurance programs are embedded in all SP activities, and TIR99 provides recommendations for a quality assurance program for probes and dilators. More specifically, the guidance identifies performance measures and process monitors that can be used for continuous quality improvement (CQI) programs for the entire process. This includes a CQI matrix that describes the visual observations and fundamental process that selected personnel should perform as part of the quality improvement process within their areas. Performing a risk analysis is part of a quality assurance program. Risk analyses are performed to determine gaps in practices and processes that are harmful to patient safety.
TIR99:2024 is available for purchase at www.aami.org (under the “store” tab).
Susan Klacik, BS, CRCST, FCS, CHL, CIS, AAMIf, serves as a clinical educator for the Healthcare Sterile Processing Association (HSPA).
Are Your Endoscopes Clean? Tapping the True Power of Visual Inspection Tools
By David Taylor, MSN, RN, CNOR
This article originally appeared in the May 2024 issue of Healthcare Hygiene magazine.
Outbreaks of endoscope-related infections represent a global problem, and the clinical implications are sobering. Numerous peer-reviewed investigations have linked various problems, including infections and deaths, to visibly contaminated or damaged endoscopes.1-6
The Association for the Advancement of Medical Instrumentation’s (AAMI’s) revised standard, ANSI/AAMI ST91:2021 Flexible and sim-rigid endoscope processing in healthcare facilities, underscores the critical importance of proper cleaning, and important new updates and recommendations are incorporated. The previous version of the standard had a conditional recommendation that internal channels may be inspected with a borescope. The 2021 version features stronger language, recommending the use of a clean borescope to visually inspect accessible channels of flexible endoscopes before sterilization or high-level disinfection (HLD).
Common problems associated with the internal channels during endoscope processing (e.g., gastroscopes, colonoscopes, duodenoscopes and more) include but are not limited to:
- corrosion
- cracks and scratches
- other physical damage
- debris
- discoloration
- moisture
- foreign objects (e.g., lint, fibers, brush bristles and particles, and clips)
The use of a small-diameter borescope for inspecting internal channels should be part of a comprehensive visual inspection program to identify internal defects, damage or bioburden that would otherwise go undetected. Small-diameter borescopes serve as way to examine the full length of each channeled lumen. Not only does that help protect patients but also provides critical insight into an endoscope’s condition, which can prevent further damage, costly repairs and premature device replacement. Note: Visual inspection should include outside examination of the device with a magnifier or microscope (ideally, with lighted magnification), with internal channel inspection performed with a borescope.
It is recommended that the person performing the borescope inspection be adequately trained and competent in the task. Use of the borescope should always be aligned with the borescope manufacturer’s instructions for use (IFU). Healthcare organizations should identify high-risk flexible endoscopes, such as duodenoscopes, bronchoscopes, ureteroscopes and cystoscopes, and ensure they undergo appropriate cleaning verification testing after each use. The test results should be consistently recorded and maintained.
If an endoscope does not pass the cleaning verification test, the endoscope should be re-cleaned and re-tested. If the device repeatedly fails cleaning verification testing, it should be removed from service and clearly labeled as needing repair to prevent further use. Damaged endoscopes, along with their unique device identifier and patient identification information, should be reported to Infection Prevention and Quality personnel, in accordance with the organization’s policy.
In conclusion, borescopes are useful tools for inspecting endoscope channels and interior components and detecting debris and other flaws that otherwise would likely be undetected; however, it is essential that those responsible for visual inspection receive proper training and education to not only use the tools effectively, but also understand the defects detected and how to address them. Using quality visual inspection tools to identifying issues with endoscopes and other lumened devices, such as residual bioburden and cracks, scratches and other problems, is a prudent practice that will greatly improve patient safety and outcomes and reduce the risk for costly device damage, malfunction and, above all, infections.
References:
- Ofstead CL, Hopkins KM, Eiland JE. Borescope inspection of endoscope working channels: Why and how? Endosc Int Open. 2022 Jan 14;10(1):E109-E111. doi: 10.1055/a-1512-2813. PMID: 35047340; PMCID: PMC8759935.
- Ofstead CL, Hopkins KM, Smart AG, Eiland JE, Wetzler HP, Bechis SK. Reprocessing Effectiveness for Flexible Ureteroscopes: A Critical Look at the Evidence. Urology. 2022 Jun;164:25-32. doi: 10.1016/j.urology.2022.01.033. Epub 2022 Feb 3. PMID: 35123986.
- Ofstead CL, Buro BL, Hopkins KM, Eiland JE, Wetzler HP, Lichtenstein DR. Duodenoscope-associated infection prevention: A call for evidence-based decision making. Endosc Int Open. 2020 Dec;8(12):E1769-E1781. doi: 10.1055/a-1264-7173. Epub 2020 Nov 17. PMID: 33269310; PMCID: PMC7671768.
- Galdys AL, Marsh JW, Delgado E et al. Bronchoscope-associated clusters of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2019;40:40–46.
- Rauwers AW, Troelstra A, Fluit AC et al. Independent root cause analysis of contributing factors, including dismantling of 2 duodenoscopes, to an outbreak of multidrug-resistant Klebsiella pneumoniae. Gastrointest Endosc. 2019;90:793–804.
- Kumarage J, Khonyongwa K, Khan A et al. Transmission of MDR Pseudomonas aeruginosa between two flexible ureteroscopes and an outbreak of urinary tract infection: The fragility of endoscope decontamination. J Hosp Infect. 2019;102:89–94.
Sterile Processing Leaders: Are You Keeping Track of IFU and Manufacturer Updates?
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the April 2024 issue of Healthcare Hygiene magazine.
Today’s medical devices are increasingly sophisticated and complex, and with up to thousands of items being processed through the sterile processing department (SPD) each day, it is essential that sterile processing (SP) professionals have access to—and diligently follow—manufacturers’ most current instructions for use (IFU) and other pertinent updates about their products.
As a consultant who travels the country to assist with SP operations, I often witness departments that are not keeping up with their IFU. As a result, they are either processing devices incorrectly or devices are being used for a purpose other than their intended and approved purpose. When I have questioned these practices, the answers are typically the same: “We have always done it that way” or “We didn’t know we were using it incorrectly.”
Here’s just one example: Nearly nine years ago, Parks Medical Electronics Inc. issued a letter to their customers outlining the appropriate use of their doppler probes. The items are classified by the U.S. Food and Drug Administration (FDA) as Class II medical devices (noninvasive), which are deemed moderate to high-risk. Class II devices are not for subcutaneous use (under the skin). Despite the manufacturer’s notification, nearly every hospital I have visited still uses these devices inappropriately. More specifically, I have witnessed the probes being used for vascular, cardiac and urological procedures—not their intended purposes.
Of course, the probe misuse is just one example of many. Many facilities use needle holders as pliers, for example, to pull pins and twist wire, which can cause cracks and render the instruments nonrepairable.1 There are other incidents where recalled products are still in use. Whatever the case, misusing devices and equipment poses a significant safety risk to users and patients. It can also void manufacturer warranties, cause premature product replacement, and increase liability for the healthcare facility.
Understand and follow device classes
In 1939, Earle H. Spaulding devised a rational approach to disinfection and sterilization of patient care items and equipment. His deep study of disinfection and sterilization refined the classification of appropriate treatment of medical devices based upon how the devices are to be used. He proposed a strategy for sterilization (or high-level disinfection) of inanimate objects and surfaces based on the degree of risk involved for their medical use. Known as the Spaulding Classification, the risk-based system includes three categories for medical devices: critical, semi-critical and non-critical.2
Critical items are equipment and instruments that penetrate the skin or enter sterile tissue or the vascular system. They can include surgical instruments, cardiac or urinary catheters, implants, and ultrasound probes. These items require sterilization (i.e., steam, ethylene oxide, hydrogen peroxide gas plasm). Semi-critical items contact mucous membranes or nonintact skin. This category can include respiratory therapy and anesthesia equipment, some endoscopes, laryngoscope blades, esophageal manometry probes, cystoscopes, among other devices. At minimum semi-critical items require high-level disinfection (HLD) using chemical disinfectants (i.e., glutaraldehyde, hydrogen peroxide, ortho-phthaladehyde, or peracetic acid with hydrogen peroxide). Note: Because of device complexity, sterilization is being recommended for more semi-critical devices.
Non-critical items are those that only contact intact skin (not mucous membranes) and pose the least risk for infection transmission. This category can include items such as blood pressure cuffs, stethoscopes, bedpans or crutches. In most cases, cleaning followed by disinfection with an Environmental Protection Agency-registered, hospital-approved disinfectant is sufficient for processing.
Understanding device classification helps provide the “why” behind the IFU. The IFU, of course, will reflect the device classification and should clearly outline how to handle, disassemble, inspect and process the item. If product-related updates occur, customers should receive updates directly from the manufacturer—and those should then be promptly reviewed and relayed to all SP professionals. Any questions about the revised IFU or other manufacturer notification about their devices, equipment, supplies or services should be shared directly with the company for clarification.
There may be times when a notification or update from the manufacturer is missed or accidentally overlooked. Any time a vendor representative enters the department for education or support, it is prudent to ask about any important updates or changes they can share. If IFU have been updated but those changes weren’t received, be sure to ask for a copy of the revised material. If further guidance or clarification is needed, SP leaders can ask their vendor rep to provide targeted education to address concerns and ensure all technicians understand the recommendations.
To ensure that devices and equipment are used correctly and safely, manufacturers regularly update their IFU and, at times, may issue customer notifications to alert them to changes, concerns or other new information about their products. All devices being processed, regardless of how long they have been in use, may require changes to the way they are used and managed. Therefore, SP leaders must also ensure that technicians across all shifts have access to and understand all IFU updates—just as is necessary for the latest standards, guidelines and best practices.
Healthcare organizations are responsible for ensuring their patients receive the safest, highest quality care. Therefore, it is imperative that devices are managed and processed consistently according to each manufacturer’s current IFU. Patients expect clean, sterile, safe and well-functioning devices, and surgeons and other clinicians expect that instruments and equipment coming from the SPD will meet those critical expectations. SP professionals must always remember that when releasing a device or instrument set for use, they are essentially stating to their end users that the items are safe for patient use. Following IFU to the letter and seeking guidance from the manufacturer whenever any recommendations are unclear are among the best ways to ensure they are indeed safe.
References
1. Schultz, R. Scissor and Needle Holders 101. PROCESS. January/February 2020. Pp. 82-84. HSPA.
2. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). A Rational Approach to Disinfection and Sterilization. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html#:~:text=Spaulding%20believed%20the%20nature%20of,in%20use%20of%20the%20items.
David Taylor III, MSN, RN, CNOR, has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019. He is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas.
Run SPD Like an Efficient, Effective Business
By David Taylor III, MSN, RN, CNOR and Robert Leenan, BS, CRCST, CIS, CCSVP
This article originally appeared in the March 2024 issue of Healthcare Hygiene magazine.
Sterile processing (SP) professionals help ensure quality patient care, infection prevention and safety through the provision of clean, well-functioning, sterile and on-time instruments. In fact, the role successful, effective and efficient sterile processing departments (SPDs) play in day-to-day business operations can be a strategic differentiator, allowing organizations to boost outcomes, reduce costs and gain a competitive advantage.
Every department within a hospital or health system plays a key role in the financial success of the organization; therefore, each department is like running a small business within a business.
To run the SPD like the business it is, leaders should assess how they provide the highest quality products and services consistently. As consultants, we often ask ourselves how SP leaders can be more strategic—effectively managing the core of their business while remaining open to necessary changes and adapting to new standards, products, equipment, policies and more. It is important for SP leaders to ask: Would the work our SPD produces be competitive in an open market? The best SP leaders show up on time, set goals for themselves, their employees and their departments, and plan diligently to create an operational structure that produces consistent, high-quality products and service.
Metrics and standard work
The cost of production and ability to adapt—all while providing a consistent product—is vital for the success of any business, including a healthcare department like the SPD. Paying close attention to the metrics relevant to the SP discipline is prudent; without metrics, it is impossible to know whether operational functions being measured yielded the intended result (quality).
Understanding the importance of quality and having the knowledge and ability to optimize costs and process outcomes are critical for increasing workflow efficiencies across device processing functions. Equally essential is understanding the impact of proper labor utilization and equipment capacity, setting time and quality expectations, and ensuring adequate resources to meet customer requirements—all variables that help the SPD provide the highest quality products, without compromising integrity. The bottom line: If one knows how to control costs and understands the variables to those costs, they can better control quality outcomes.
When improving SPD workflow, two factors must be considered. First, it is necessary to create standard work for each process while incorporating quality steps into that standard work. Next, leadership standard work should be established and comprised of tasks and routines that support conformity to each process. Standard work is defined as the documented steps for performing a job task and outlines who is going to perform the task as well as when and how. Note: In the absence of this standard work guidance, a veteran employee with decades of experience could easily introduce their personal preferences, leading to variations and inefficiencies that cost significant time and money and negatively affect quality.
Applying lean methodology can help businesses drive efficiencies in some or all operational areas by finding and eliminating wasteful, non-value-added steps within processes. Waiting for something that is delayed or requires reworking can be extremely frustrating for SP and end-user employees. Frustrations become further magnified when the challenge could have been avoided. A lean memory device acronym, known as TIMWOODS (Transportation, Inventory, Motion, Waiting, Overprocessing, Overproduction, Defects and Skills) can help identify eight wastes. In everyone CSPD’s processes, one or many of these inefficiencies can add up, creating unnecessary work to complete a task.
Note: See the March issue for Figure 1: CLICK HERE to access.
The difference in time standards and what is lean (and not lean) can be attributed to all types of wasted effort. In the SPD, handling items multiple times, having to perform rework due to defects, failing to complete work correctly, and misplacing trays and instruments that require multiple staff members to search for them are all common examples.
Leaders cannot assume that all employees will consistently follow process standard work. There may be valid reasons for deviations at times; however, without a standardized process, leaders cannot effectively evaluate or control quality. Leadership standard work helps ensure compliance, monitor effectiveness, identify improvement opportunities, and implement countermeasures.
Again, using Figure 1 as an example, using 300 trays processed in a 24-hour period would add 6.0 full-time equivalents (FTEs) to the budget to complete the same amount of work.
Leaders can set expectations with frontline leaders allowing them to manage those expectations with their staff. With a standardized process and effective communication, SP leaders can expose weaknesses in their operations. For example, it could take 20 minutes of actual work to assemble one tray and become part of the planned process. Whatever the starting point, identifying all forms of waste and eliminating it will lead to improvement. When SP leaders reach this point of the process, they have achieved the initial steps of establishing a continuous improvement structure for their department. In time this structure will keep identifying opportunities, and leadership will implement countermeasures to identify all waste in the process. This will be comprised of all value-added steps or essential steps for this process—and when that process is multiplied by the number of instruments and trays in one’s operations, the benefits will be significant.
SP leaders are required to balance consistency and responsiveness to run their business successfully, safely and efficiently. It is essential to define, measure and improve quality of services by utilizing processes, standard work, and data to influence positive outcomes.
David Taylor, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and principle of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019.
Robert Leenan BS, CRCST, CIS, CCSVP is a sterile processing improvement consultant and managing partner at SPD Solutions LLC.
Preventing High-Risk Exposures in Sterile Processing
By Tony Thurmond, CRCST, CIS, CHL, FCS
This article originally appeared in the February 2024 issue of Healthcare Hygiene magazine.
The patients and healthcare workers Sterile Processing (SP) professionals serve each day rely on clean, high-level disinfected or sterilized medical devices and equipment. Many, however, are largely unaware of the routine dangers and risks SP professionals are exposed to in the departments in order to provide those necessary services.
During a typical workday, SP technicians face risks from chemicals, bloodborne pathogens, excessive temperatures, sharps exposure, musculoskeletal injuries and slip-and-fall risks, among other dangers. Each can be detrimental to employee health and safety and, potentially, even to subsequent patients. Fortunately, such incidents can be mitigated with collaboration and broad-scale involvement from the SP team and, as appropriate, interdisciplinary teammates. Medical facilities have an obligation to keep employees safe, but it is also up to each departmental leader to train in safe practices and ensure adherence to them.
Documenting competencies
The decontamination area poses the greatest risk for SP employees. The Occupational Safety and Health Administration’s (OSHA’s) Bloodborne Pathogens Standard (29 CFR 1910,1030) requires that each facility has an exposure control plan that outlines the potential dangers that employees may encounter on the job. When performing decontamination duties, employees must wear appropriate personal protective equipment (PPE) that includes gloves, a gown or jumpsuit with sleeves and a fluid-resistant barrier, fluid-resistant shoe covers and mask, and eye-protection (goggles or a face shield) that protects from splashes. OSHA requires these items to be provided by our facilities for employee protection.
Every SP professional must fully understand how to use chemicals safely and effectively in the department, and they must also know the risks involved when used incorrectly. While these products may do a great job in cleaning and removing soil from instruments, they can be dangerous if used improperly or when a spill occurs. A safety data sheet (SDS) must be readily available to technicians to address spills or other exposures. Most facilities have SDSs stored electronically on workstation computers, while others may depend on a written copy of the SDS to guide them. All SP employees must know where the SDSs are kept and be able to access them readily.
SP professionals should also receive initial orientation of the required PPE for their department, and also know the proper policies and procedures for handling workplace exposures. This includes documented orientation, continued education offerings and a competency to physically demonstrate the necessary actions if an exposure occurs. Continuing education and reviews or competencies should be reviewed periodically and documented for each employee.
Addressing eye and sharps risks
Every SP employee must know where the eye wash station/shower is located and how to use it correctly. There are specific requirements for eye wash stations set forth by the American
National Standards Institute, including the need to have an eye wash/shower available within 10 seconds of travel time, with unobstructed access for areas where potentially dangerous chemicals are used. Eye wash sinks should not be a sink used for decontamination. According to ANSI/
ISEAS Z358.1, they should be tested at least once a week and for an established period to verify operation and ensure the flushing is lukewarm (between 60 and 100 degrees Fahrenheit or between 15 and 43 degrees Celsius). This routine testing should be documented.
Statistics show that 25 percent of healthcare worker sharps exposures happen to support staff such as SP professionals. Most sharps-related injuries are documented in the operating room (OR) from cuts with the scalpel or needlesticks. SP professionals who have worked in the discipline for any length of time have likely seen a sharp come to decontamination from surgery alongside the soiled instrumentation (such as a knife blade, suture needle, drill bit, saw blade). SP leaders should communicate such incidents with those in the OR so they understand the risks.
Handling instruments in decontamination creates opportunities for injuries as well. At times, items used during procedures are placed in the case cart in an unsafe manner, which introduces the opportunity for injury. It is important to never reach into a pile of mismanaged, disorganized instruments. It is also important to lift each instrument tray individually and always use the handles of the tray. As each tray is removed, it is essential to visually inspect the tray to look for missed needles, blades or instruments protruding from the tray.
Other departmental areas, beyond decontamination, can pose notable employee risks as well. When running the test for sterilization cycles, technicians must wear protective PPE while activating the biological test ampules. Changing the cartridge or bottle for low-temperature sterilizers may seem like a simple task, but also can introduce the risk for dangerous exposure; therefore, proper gloves, masks and eyewear are essential (and required).
Additionally, to prevent unnecessary exposure to contaminated devices, case carts must be designated as “clean” or “soiled.” If a case cart arrives in the SPD unmarked, the contents inside should always be considered soiled.
Preventing workplace exposures is everyone’s responsibility, from SP professionals to the surgeon, surgical tech, OR nurse, transporter and employees in other procedural areas of the facility. Ongoing communication and education are critical in keeping the SP professionals and others safe from avoidable harm.
Tony Thurmond, CRCST, CIS, CHL, FCS, is a past-president of the Healthcare Sterile Processing Association (HSPA) and an HSPA columnist. He serves as sterile processing manager at Dayton Children’s Hospital.
Measuring SPD Productivity Vital to Safety, Quality
By Tony Thurmond, CRCST, CHL, CIS, FCS
This article originally appeared in the January 2024 issue of Healthcare Hygiene magazine.
Many sterile processing (SP) professionals understand the frustration of giving their best effort every shift while still failing to meet daily demands. Adding to that struggle is that some SP leaders struggle with how to measure productivity effectively, especially when there are opposing views about what productivity means. Often, productivity is tied to profit or loss—with the focus on output instead of actual demand for products and services. This is challenging because an employee or team can be productive, even if they struggle to meet customer demands and requirements. Put simply, a technician might feel satisfied by their efforts and output but still fail to keep up due heavy procedural volume and limited instrumentation and other resources.
Certainly, productivity varies across departments and disciplines, but understanding the basic definition will serve as an essential first step in establishing a plan to measure it. Merriam-Webster defines productivity as the quality or state of being productive and the effectiveness of productive effort, especially in industry, as measured in terms of the rate of output per unit of input. Using that definition, SP productivity would calculate the number of products produced as well as all tasks associated with their production.
When assessing SP productivity, surgical volume and each associated task should be measured.
Many organizations measure SPD productivity solely on surgical case volume—an outdated method that leads to inaccurate calculations. A base rate must be factored for each patient and procedure. A minor procedure requiring only two instrument trays, for example, will require less productivity than is required to process devices for a total hip revision.
Individual tasks should be defined, and a time study should be performed and the degree of difficulty and possibility for interruptions should also be factored into the measurement. When timing the decontamination process for robotic instruments, it would be necessary to consider the length of time required to correctly clean and disinfect the device and any attachments according to the instructions for use (IFU). Routine tasks, such as answering phone calls, emptying the cart washer, or delivering an item to the procedural area, must also be calculated using a time study to determine averages. It is important to capture true minutes spent on various tasks. For example, delivering items to one user area may take longer than another due to the physical distance from the SPD.
Every task should be included that captures the team’s daily efforts. Author’s note: In my facility, we list our breaks and lunches and time spent restocking workstations, answering the door or phone, changing detergents, filling supplies, emptying washers, and the list goes on. Each task is measured and documented. Time studies should also be conducted to assess the length of time needed to process trays from decontamination and preparation to their delivery at the point of use. Tracking productivity metrics also requires consistent input and compliance from all SP staff members, and SP leaders should provide data to demonstrate metrics, improvements, and opportunities for change. Instrument tracking systems can be invaluable for automatically capturing productivity metrics. Employees should be trained to input tasks as they are performed, and this productivity data will be essential for leaders to share with facility executives. SP leaders are often asked to justify their staffing, even when replacing a budgeted position. Some organizations collaborate with consultants to further reduce labor costs—the primary expense for healthcare organizations today.
Recently, when such a consultant visited our SPD, I asked which metrics they needed. I showed the tasks we perform daily, along with the average time and employees needed to complete each task properly. I was prepared to show productivity for each area, from decontamination through sterilization, distribution and storage. Even after showing our productivity, the consultant focused on the number of employees staffed in each area of the department. Without considering any of the key metrics we documented and shared, the consultant then questions why we needed two to three people working in decontamination. I explained that several items being decontaminated require timed cleaning processes that involved far more than simply spraying water and running the devices through the washer. I also stressed that each instrument is washed manually and inspected, and that the tray must be organized so the washer’s manifold arms can effectively clean what may have been missed during manual cleaning—all steps that require added time and employee focus. Finally, we shared the IFU, which further justified our resource requirements. The consultant acknowledged their lack of understanding of how time and resource needs are best calculated and seems impressed by the level of detail and metrics we provided to support our requested needs.
In conclusion, capturing true productivity offers significant benefits for all SPDs. SP leaders should work with their teams to assess productivity and resource requirements that will help ensure the departments have what they need to meet customer demands safely, consistently and in accordance with IFU, and the latest standards, guidelines, and best practices.
Tony Thurmond is the central service manager at Dayton Children’s Hospital in Dayton, Ohio. He is also a columnist for the Healthcare Sterile Processing Association (HSPA), past-president and board of director member who currently serves on HSPA’s Editorial and Fellowship Committees.
Securing Funds for SPD Capital Purchasing to Help Keep Operations Running Optimally
By Marie Brewer, CST, CRCST, CIS, CHL, CER, GTS, CLSSBB
This article originally appeared in the December 2023 issue of Healthcare Hygiene magazine.
Sterile processing (SP) leaders often experience budgetary cuts or see their requests for capital purchases denied, but tapping alternate sources of funding can help bridge the budgetary gaps and set up the Sterile Processing department (SPD) for greater operational success, safety and efficiency.
Grants are an excellent option for obtaining critical funding, and learning to effectively and efficiently write a grant proposal is the secret to success. There are several common approaches to grant writing, including:
• Letter of inquiry (LOI) – An LOI is a brief letter written to discuss the main points of a full proposal; this allows the funding organization to determine if the request meets their grant criteria. Some facilities use an LOI as the first step toward obtaining funding for capital purchases.
• Full proposal: This approach follows a standard format (as outlined by the funder) that typically includes a cover letter, statement of need, project summary and budget. A full proposal can range from five to 25 pages.
• Letter proposal: This format may be used to propose a scholarship for education or capital equipment requests. Often, a two– to four-page letter is sufficient. The letter should describe the team or organization, outline the detail(s) of the project and define the sponsorship proposal.
Note: To ensure the best outcomes, SP leaders should research each prospective funding organization. Most funders have guidelines for eligibility and will provide their preferred proposal format and a list of required documentation or supporting material. They are also likely to state their submission deadline(s) and general timelines for notification and grant payment.
This article focuses on letter-writing because it is the method that has garnered me and my department the most success. To date, through our grant writing, we have received more than a dozen awards for capital items, such as borescopes, height-adjustable workstations and sinks, and additional surgical instrument sets to improve processes, safety and efficiency. The smallest grant we received was $2,000 for education-based scholarships, and the largest exceeded $75,000 for height-adjustable sinks. In 2022, our department received a $15,000 grant for a new height-adjustable workstation. Additionally, educational scholarships and grants have funded trips for several technicians to attend the Healthcare Sterile Processing Association (HSPA)’s annual conference.
Grant-proposal writing tips
Before starting the letter-writing process, SP leaders should work to avoid common mistakes that can derail grant attainment. Some examples include repeating exact phrases from the funder’s guidelines, failing to read and understand the grant guidelines, using industry jargon that may not be understood by grant-funding personnel, focusing more on problems than solutions (or suggesting general solutions to specific problems), submitting the letter after the stated grant proposal submission deadline, and submitting the letter with typos or insufficient or inappropriate information.
Before writing and submitting a letter proposal, SP leaders should carefully review the grant’s requirements to determine eligibility and ensure the letter is focused and tailored to the audience. It is vital to always proofread the letter to ensure the content is concise, easy to understand and clearly advocates for the SPD’s needs by laying out the specific goals and needs. Finally, define the budget accurately to ensure all associated project costs are included.
Prior to writing the official letter proposal, prepare a grant letter outline and include these core elements: Briefly introduce your organization (health system, hospital, surgery center, etc.); tell the funder how much money is being requested by why; succinctly explain what sterile processing entails and describe the work performed by technicians in the department; and include research-based content and data to support the team’s needs and funding request.
When writing the grant letter, it is best to start by briefly explaining how securing capital needs will advance the quality of patient outcomes and employee safely and satisfaction. In my own grant-writing experience, my letters include a description of our department, including the number and type of staff and the target population we serve. Also included is some simple data such as the number of annual admissions or patient visits the department supports, along with an explanation about the project or program, an identification of the specific need(s) and how the grant will support those needs. A project timeline is also included as is justification about how the project aligns with the mission or the funding organization. Finally, be sure to provide an explanation about what the funds will cover. For capital equipment, include a description and photograph of the equipment, how often the equipment will be used, and the life expectancy of the equipment (include warranty information). Also, provide a detailed budget (consider incorporating accurate figures/data into a table or chart), and a detailed quote and bids for additional installation or renovation costs.
Following the writing of the grant proposal, ask two or three independent readers to review the writing critically to ensure it is easy to understand, accurate, error– and typo-free, compelling and meets the funder’s guidelines and requirements.
Grant writing can be an intimidating process for SP leaders, especially those who are new to the process. It is, however, a practice that can be learned and mastered and lead to notable departmental improvements that can increase process safety, efficiency and efficacy. The more grant letters are written, the more proficient leaders will become at the practice. Even a denial from a funding organization can provide valuable lessons to help sharpen and perfect future grant-writing attempts.
Marie Brewer, CST, CRCST, CIS, CHL, CER, GTS, CLSSBB, serves as sterile processing manager at St. Luke’s Hospital in Cedar Rapids, Iowa. She has served as a columnist for the Healthcare Sterile Processing Association (HSPA) since 2022.
Instrument Maintenance: Inspected, Tested and Repaired
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the November 2023 issue of Healthcare Hygiene magazine.
With shrinking healthcare budgets and the ongoing quest for infection prevention and the delivery of safe, high-quality patient care, the need to effectively maintain (and sustain) a robust and proactive instrument maintenance and repair program has become even more critical. More specifically, it is imperative that all sterile processing (SP) professionals recognize the importance of proper device care and handling, cleaning, inspection and testing of all items they manage and process.
Having a well-educated staff that knows how to properly manage instruments and identify issues before they become bigger problems is key to a successful instrument maintenance program—and to healthcare organizations’ broader quality, infection prevention and safety initiatives. The delicate nature of surgical instruments and devices and the high cost associated with preventable damage and subsequent repairs and replacement warrants focused training and attention by SP technicians as well as the surgeons, nurses and other staff members in procedural areas.
Aligning resources
Having adequate instrumentation inventories, time and other resources to meet procedural volume —and ensuring that devices are processed and managed according to instructions for use (IFU), standards, guidelines and internal policies and procedures — is critical; however, in my consulting experience, I have seen many facilities fail to invest in adequate instrumentation to support their surgical programs, which results in device overuse and reduced life expectancy for instrumentation.
Every device being reprocessed should undergo thorough inspection before being packaged for reuse or storage. The importance of thoroughly inspecting each instrument cannot be overemphasized. Instruments are subjected to a great deal of abuse over their life cycle, and repeated use and rough handling can damage instruments over time, increasing the risk for device failure during the procedure and, potentially, device contamination that may cause patient infections.
The following steps should be performed routinely by SP technicians:
• Hinged instruments (single or double) should be checked for cleanliness and stiffness (they should move smoothly and easily). Ensure that jaws and teeth are in proper alignment.
• Box locks, serrations, and crevices should be inspected for proper function and cleanliness. Bioburden and other debris can significantly impact the proper function of the box lock function.
• Instruments with cutting edges, such as scissors, rongeurs, chisels, curettes, and the like, should be checked for sharpness. Cutting surfaces should be straight and aligned and absent of nicks, chips or dents.
• Ratcheted instruments should open and close easily. When closed, they should hold firmly.
• Instruments with pins or screws should be carefully inspected to ensure they are intact and functioning as intended.
• Plated instruments should be inspected for chips, worn spots or sharp edges. Defects in the plating can harbor debris and bioburden (and rust over time).
In-house or outsourced?
There are two options when establishing an instrument maintenance and repair program. The first is to create an in-house program, employing a skilled instrument repair technician (or technicians) to work for the SPD/organization. This option may not be financially feasible for a small- or medium-sized facility; however, it may be better suited to large hospitals and health systems.
The second option is to outsource the program. Many third-party companies provide instrument repair and maintenance programs (and, of course, original equipment manufacturers also service their own equipment*). It is essential to investigate the quality and depth of the services provided and ensure that their technicians are skilled and well educated—and then take time to compare the various options and service providers. Remember that the least expensive option is not always best. Also, before choosing a maintenance and repair provider, it is prudent to understand the current state of existing instrumentation by completing a thorough assessment of all devices and equipment in inventory. This process will ideally include:
• Assessing current instrument inventory and comparing it to the number of specialties each instrument set supports. Do you have enough instruments (loose or in sets) to support your surgical program?
• Asking how many times an instrument set has been reprocessed in a day to meet the needs of the operating room (OR). Does current inventory support the workload?
• Evaluating the current condition of the instrument inventory.
• Identifying which devices have been repaired previously. If they were repaired, how many times?
• Understanding the rotation cycle (weekly, monthly, quarterly) for each instrument set to be inspected, repaired if necessary, and returned for reprocessing.
• Tracking and documenting repairs.
• Assessing the current repair and maintenance process and asking what is and is not working well for the SPD and its customers.
• Ensuring that SP staff and end users have knowledge about how to safely handle devices and identify issues proactively instead of reactively.
Completing the assessment will help determine whether an in-house maintenance and repair program is feasible or whether an outside provider is the better option. Before selecting a vendor, it is advised to seek competitive bidding (again, being careful to weigh the cost estimates with the precise services that will be provided). Bids may include the initial evaluation and repair of all instruments, with ongoing routine maintenance. Whichever program is chosen, the repair technician(s) should be able to provide ongoing education and preventive maintenance training as part of their employment or contracted service.
The cost of properly maintaining instruments is far less than that of procedural delays, extensive repairs and premature replacement of devices—and above all, negative outcomes for the patient. A proactive instrument maintenance and repair program helps prevent issues before they arise (or develop into more extensive damage that requires costlier repairs, downtime or premature device replacement). Organizations that proactively adopt a robust instrument maintenance and repair program directly and positively impact productivity, customer satisfaction and profitability.
*Be sure to carefully read manufacturers’ warranties and contact the manufacturer if further clarification is needed. Some device and equipment manufacturers will void warranties if their products are repaired by another party.
David Taylor III, MSN, RN, CNOR, has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019. He is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas.
Sterile Processing Leaders, Do You Know How ST108 Affects Device Processing?
By Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS
This article originally appeared in the October 2023 issue of Healthcare Hygiene magazine.
It is essential that sterile processing (SP) professionals have ready access to the latest standards and guidelines and work to ensure that recommended best practices are understood and consistently followed. One of the most anticipated standards to be released was ANSI/AAMI ST108:2023 Water for the processing of medical devices, an upgrade from the water quality technical information report TIR 34.
Water is a primary resource used for medical device processing, relied upon heavily in the cleaning process and to generate steam for sterilization. The risks associated with poor-quality water being used on a medical device during processing can result in ineffective cleaning and disinfection, device malfunction during use, toxic residues remaining on a device, patient infection, and pyrogenic reactions. ST108 addresses various levels of water quality and steam purity suitable for medical device processing and describes water treatment processes that can be used to produce water that meets quality requirements for medical device processing. It is an especially valuable resource because it addresses water quality at the point-of-use and for cleaning, rinsing, disinfecting and sterilizing medical devices.
Water-type differences
ST108 defines three types of water quality and provides the specific performance
qualification levels of water quality for each. All levels are required to meet the water quality
measurement values as defined in this standard. The three levels include:
Utility water—Water that comes from the tap and is the type recommended for cleaning
medical devices. It can also be used for the initial rinse to remove soil loosened by the cleaning
process and for removing cleaning agent residues. This water may require further treatment at
the facility to achieve the specified water quality. This water is mainly used for flushing,
washing and intermediate rinsing (rinsing between cleaning and disinfection). Note: Tap water may not meet the requirements of utility water as defined in ST108. For that reason, tap water used in SP areas should be analyzed to determine its characteristics and whether treatment is needed to meet the requirements for utility water. The water treatment system manufacturer should be consulted to validate that the treatment process will be adequate for the specific characteristics of the incoming tap water.
Critical water— Water that meets the water quality measurement values as described in
AAMI standards. To achieve these values, water generally requires a level of purification that
can only be achieved by removing ionic contaminants from the water (this process is referred to as reverse osmosis or deionization). There are instances where both technologies may be used together to meet the water characteristics needed to be classified as critical water. There will also be other forms of treatment that can be found with systems that produce critical water. Softening, carbon filtration, coarse filtration, ultrafiltration, UV lighting are some of the technologies that could be found with these systems. This water is mainly used for the final rinse after high-level disinfection and/or for the final rinse for critical devices prior to sterilization. For automated endoscope reprocessors (AERs), it is important to understand the equipment IFU to ensure equipment compatibility with critical water. Note: Much of today’s equipment is not designed to handle the corrosive nature of critical water; therefore, utility water is specified for the final rinse.
Steam—Vaporized water produced by a centralized boiler or a generator/heat exchanger near the
sterilizer. Most steam used in a Sterile Processing department (SPD) is piped from a centralized system for many other purposes (known as plant or house steam). The steam is generated in this
centralized system and transferred through piping to the sterilizer. Other types of steam are
located close to the sterilizer; based on the design of the equipment and the respective IFU,
utility or critical water may be used in a pure steam generator.
Water quality for SP equipment, core functions
ST108 provides water quality factors for washer-disinfectors. Washer-disinfectors are often
equipped with at least one control valve for hot water and another for cold water. A separate
control valve is for the final rinse.
Ultrasonic (sonic) cleaners are also useful for removing soil from joints, crevices, lumens and other areas that are difficult to clean by other methods. Utility water can be used unless otherwise indicated by the ultrasonic or medical device manufacturer’s written instructions for use (IFU). The key water-quality factors to consider for ultrasonic cleaning include water hardness and physical appearance (color, clarity and absence of particulates/sediment). If the ultrasonic cleaner does not provide a final rinse, the device manufacturer’s written IFU for manual rinsing of the device should be followed.
Routine monitoring is necessary to ensure that the water quality is maintained and does not deteriorate over time. If water quality is not monitored, the water treatment storage and distribution system could become heavily contaminated with metals, microorganisms or other contaminants and could contribute to corrosion, staining and increased microbial levels after processing. ST108 provides guidance on what requires monitoring, how often it should be performed and by whom; the standard features a chart to outline those functions. Utility water in medical device processing should be monitored quarterly at each point-of-use location, such as a sink, by collecting a water sample in a specially designated collection cup that is then sent to a lab for analysis, with a daily visual inspection of the interior of processing equipment to check for residues, staining, scaling, and discoloration.
Critical water requires more frequent monitoring; if not properly maintained, water treatments can result in heightened microbial levels, biofilm development and endotoxins. In addition, the making of critical water will affect the chemical attributes of water including the pH, conductivity, total alkalinity, and total hardness. As such, point-of-use critical water should be tested monthly for endotoxins and bacteria by submitting a water sample. Daily visual inspections should also be performed that include inspecting the interior of processing equipment to check for residue, staining, scaling and discoloration.
ST108’s annexes A through I provide information to assist SP professionals and others with the understanding and implementation of the standard. The annexes include guidance on the application of the normative requirements, risk analysis, automated endoscope reprocessors, water used in cleaning and moist-heat process, typical presentation of water quality issues during medical device processing, and more.
To purchase the standard, visit www.aami.org.
Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS, is a clinical educator for the Healthcare Sterile Processing Association (HSPA).
KPIs Spur Powerful Sterile Processing Department Improvement
By David Taylor III, MSN, RN, CNOR, and Kristina Pirollo-Ketchum, AA, CRCST, CHL
This article originally appeared in the September 2023 issue of Healthcare Hygiene magazine.
Healthcare organizations across the U.S. are embracing performance monitoring and improving clinical outcomes through the measured approach of key performance indicators (KPIs). By leveraging the proper technologies, data can be leveraged to develop processes that optimize patient safety through real-time management and visibility of interdepartmental workflows— within the sterile processing department (SPD) and other procedural spaces such as the operating room (OR).
Measuring and assessing achievements in the SPD is a combination of both art and science. KPI and data analysis are elements designed to track and measure work performance. Specifically, KPIs refer to a set of quantifiable measurements used to gauge long-term performance and help determine strategic, financial and operational achievements over time, and then compare that performance to regulatory guidelines and standards and others within the profession. Having access to such quality information enhances the understanding of issues affecting the SPD and empowers the departmental team to make informed decisions. Moreover, data plays a vital role in achieving quality outcomes. When defining the metrics, it is important to gather and track specific data sets to guide data collection efforts.
SP leaders and their teams should:
Identify what to measure. Understanding what needs to be accomplished in one or more areas will help SP leaders determine the metrics that align with their departmental and customer goals and objectives. These could include productivity, daily throughput, number of sets processed per employee, error rates, inventory optimization, same-day instrument turnaround times, biological monitoring or sterilization cycle rates, equipment maintenance, documentation log completeness, and infection rates. Labor time studies that accurately predict staffing and capacity needs are also beneficial, allowing SP leaders to more accurately budget for all operational needs. KPIs should be selected and adapted to the department/facility to support the strategy and objectives, and they should be specific, measurable, achievable and relevant.
Choose the data collection methods. It is essential to select a technique for collecting data. Methods can include manual recording through observation, use of an automated electronic data gathering system (software or cloud-based), or a retrospective review of documents and records annotated on data-entry forms. Keep in mind, regardless of the method used, data collection must be consistent and accurate to ensure meaningful results. Finally, data must be entered in a timely fashion and reviewed throughout the process.
Secure education and training. It is critical to determine who will collect the data (leader, staff member, outside entity) and provide the proper training necessary for those who are a part of the process. SP leaders should also consider training staff on data collection procedures so they can be a part of the process and understand the end goal. Instructions on information gathering (utilizing data collection tools and addressing challenges or queries) is essential, and the SP team will also require instruction and training on data entry and analysis.
Lean on data management systems. Whether one enters data into an Excel spreadsheet or uses an electronic or cloud-based software system, it is imperative to adopt the right solution that can effectively analyze the department’s data and help support reliable solutions. There are many manufactures that specialize in data management systems, so SP leaders should do their homework to determine the best system for their needs. Advanced data management solutions include tracking systems, data analytics platforms and specialized data-processing software. Such systems can automate data collection and allow real-time reporting for informed decision making.
Analyze and present the data. It is critical to routinely and consistently analyze the collected data to identify gaps in practices that might contribute to over– or under-work. It is crucial to identify trends and any areas or processes that are not keeping pace with workflow expectations.
It is valuable to compare and measure the data against industry standards and best practices as well as like-sized facilities. This will provide insight into how one’s own SP operations are performing. Evaluating and comparing the data will allow SP leaders to identify areas for improvement and set goals that drive improvement initiatives for their department and facility.
Once reviewed, the findings should then be shared with SP staff. Present the data in a format everyone will understand; consider using charts, graphs or dashboards that will help employees better visualize the data. Doing so will help SP staff pinpoint areas where process improvement can be made, optimize workflows, improve interdepartmental communication, and provide senior leadership with the information they need to make informed budgetary and support decisions for the SPD.
Conclusion
SPD processes are complex, often involving multiple interconnected subprocesses in order to provide procedural areas the devices and equipment needed to perform their roles. SPD processes are also closely connected to patient safety and quality of care, workflow and stakeholder satisfaction (patient, physician/surgeon, registered nurse, perioperative staff, and hospital administration).
KPIs offer SP professionals unique insights into their departmental service and operations. By determining the KPIs for each departmental area, the SPD can identify areas of improvement and make informed decisions to optimize their processes and operations. The capture, analysis and dissemination of quality data is essential, as is establishing measurable objectives to monitor progress.
David Taylor III, MSN, RN, CNOR, has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019. He is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas.
Kristina Pirollo-Ketchum, AA, CRCST, CHL, is interim leader for Novia Solutions.
KPI Data Examples for Various SPD Areas/Processes
Decontamination:
• Quality checks (e.g., tray completeness, accuracy)
• Throughput
• Instrument quality
• Tray weight
• Early release
• Cleaning logs
Sterilization:
• Equipment verification testing
• Load arrangement
• Documentation of load
• Biological testing
• Sterilization failures (e.g., time, temperature, pressure, wet loads)
• Rate of immediate use sterilization
• Cleaning logs
Storage area:
• Outdates
• Event-related issues
• Cleaning logs
• Temperature and humidity
Case cart builds:
• Number of carts completed per shift or 24-hour cycle
• Case cart accuracy
• Percentage of on-time case carts
Managing Loose Devices to Reduce Workload, Increase Throughput
By David Taylor, MSN, RN, CNOR; Kristina Pirollo-Ketchum, AA, CRCST, CHL; and Albert Huether, MBA, CRCST
This article originally appeared in the August 2023 issue of Healthcare Hygiene magazine.
Although the roles and responsibilities of sterile processing (SP) professionals can vary from one location to the next, their primary duties involve processing instrumentation and reusable medical equipment by removing gross contaminants (decontamination), inspecting and reorganizing instruments into their appropriate trays, sets and/or peel packs (reassembly), sterilizing or high-level disinfecting each item according to the manufacturers’ instructions for use (IFU); and storing that instrumentation and medical equipment for future use (sterilization and storage). Still, broken, missing or inappropriately prepared instruments—known as tray defects—can be a frequent problem for surgical and procedural teams, causing delays and negative patient outcomes.
On any given day, SP professionals can process thousands of surgical instruments over the course of a 24-hour period, depending on their organizations’ location and size. To process those items, SP technicians must work efficiently to ensure each instrument tray or set is assembled correctly for reuse. Unfortunately, many factors can impact an SP technician’s ability to perform their roles properly. For example, not all instruments are returned inside their proper tray, which can contribute to device damage and loss. Such issues must be corrected during the reassembly phase to prevent future problems for the operating room (OR) or procedural areas.
During the assembly phase, SP technicians often address issues with damaged or missing devices by replacing them with loose instruments in the organization’s inventory. These instruments are often located in the assembly areas in drawers, cabinets, containers, case carts or peg boards. Many loose instruments in inventory are disorganized, often mixed and in disarray, which makes finding a replacement instrument time consuming or, in some cases, impossible. Additionally, the condition of loose instruments may not always be much better than the devices they are replacing. Inappropriate storage practices can damage loose instruments, leading to lost items, costly replacement, and an increased risk for contamination. Redesigning loose instrument inventories can create opportunities, such as helping reduce instrument and instrument set defects, eliminating waste, over production and wasted steps, and speeding up production times.
A well-maintained physical environment is critical for managing loose instruments effectively and consistently. With demanding and sometimes daunting workloads, the last thing SP technicians need is to waste precious time searching for a device to complete a set or locating an essential instrument during an emergency. Standardization and systemization help the process and hold SP team members accountable for the work they perform.
When exploring ways to improve loose instrument inventory management, it is necessary to inventory all devices that are extra or deemed loose in inventory. Once inventoried, SP leaders can determine whether the available extra devices are enough to support the surgical specialties and procedural volume (if not, it will be necessary to budget for and purchase more). After inspection, damaged or malfunctioning instruments should be thoroughly repaired and maintained (as needed). It is important to permanently remove from inventory any instruments that are nonrepairable. Instruments of suboptimal condition that are not removed could find their way into an instrument set for patient use, adding to defect data and, most importantly, risk to patient safety.
Once all loose instruments are inventoried, purchased and/or repaired, they must be properly stored. There are multiple options for sterile processing departments (SPDs), so it is important to choose what is right for one’s own organization and to solicit feedback from employees across all shifts. Keep in mind, any storage solutions chosen must be sturdy, meet standards-based requirements and provide years of service. They must also be non-porous, so they can withstand routine cleaning with approved hospital disinfectants. Note: Storage solutions should not be comprised of wood, Masonite, cardboard or wood-based fiberboard-based product. If they are made of one or more of those materials, they must be removed and replaced with a solid, appropriate storage solution.
One method we’ve seen gaining traction in SPDs across the country is processing loose instruments in peel packs and organizing them on a peg board or in plastic bins hung on a louvered wall system. Such systems can be quite effective, while consuming minimal space. The plastic bins come in numerous colors, allowing them to be color-coded to their loose instrument inventories and alphabetized for each surgical service line. When using such a system, I suggest identifying each item in the bin with a unique code for improved identification (at minimum, consider adding a photo of the instrument, along with its name and description, manufacturer number or reorder number, and par level). Providing this information can help with location finding and reordering.
This storage approach can help SP technicians more rapidly respond to emergency instrument needs. For example, if an instrument breaks or is dropped while in use during a procedure, the SPD can immediately replace it with a sterile one by simply plucking a peel-packed replacement device from a dedicated bin. Peel packs also protect instruments from contact with other devices, which further prevents instrument damage or dulling. For SPDs that choose this method, it is essential that their leaders develop a process for routine bin cleaning (e.g., weekly, biweekly and as needed). This ensures the bins are kept clean and allows technicians to more easily inventory, inspect the peel packs for damage and restock as needed.
Whichever storage method an SPD used to manage loose instruments in inventory, it is important for supervisors and managers to maximize their employees’ efficiency and throughput, while minimizing the number of tray defects and procedural delays and improving patient safety. Disorganized storage of loose devices can diminish productivity and service quality, contribute to lost or damaged instruments, cause procedural delays, and increase costs and time associated with set reprocessing when well-functioning replacement devices cannot be located readily.
A well-designed, standardized approach to storage and retrieval of loose instruments is vital to an SPD’s ability to provide quality service to procedural areas and to ensure that all devices are processed, well-functioning and available when needed.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019.
Kristina Pirollo-Ketchum, AA, CRCST, CHL, is interim leader for Novia Solutions.
Albert Huether, MBA, CRCST, is the sterile processing department director for Medical City Dallas.
Loaned Device Management is Crucial for Patient Safety
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the July 2023 issue of Healthcare Hygiene magazine.
Loaned or borrowed instruments are medical devices owned by a manufacturer and temporarily loaned to an organization. In most instances, the loaned instruments come with little or no cost, particularly if the healthcare organization is paying for implantable devices (e.g., screws, plates, joints). The organization may agree to use the medical devices for a fixed number of interventions. If the items are loaned for several interventions or a longer period, a separate agreement—usually a consignment agreement—between the two parties is established.
Borrowing instrumentation is a common practice that can offer many advantages, including reduced costs and the ability to expand services. More specifically, borrowed specialty surgical instrumentation and subsequent implantable items can provide the much-needed inventory for healthcare organizations, without the need to purchase large quantities of expensive instrumentation to support robust surgical programs. Despite the positives, loaned instrumentation can pose numerous challenges, chief among them being hidden costs for the facility, and risks to patient safety. The costs associated with handling and processing loaned instrumentation appropriately can be substantial. Some costs can include increased staffing costs, particularly in Sterile Processing departments (SPDs). For example, when a manufacturer delivers numerous loaned trays late or unannounced, SP leaders often need to put other work on hold or use unscheduled overtime hours to process them.
Over the past several decades technological advancements have changed the face of surgery, and healthcare organizations have been forced to keep pace with those changes. One way for them to do so without adding significant costs to their bottom line is through a focused, comprehensive and effectively implemented loaned instrument program.
Follow organizational policies and guidelines
A formal and effective loaned instrumentation program hinges on having specific policies and established controls in place that allow SP professionals to effectively manage borrowed instruments and implants. Facilities should emphasize the importance of developing a standardized system that all parties (e.g., surgeons, nurses and SP staff) agree upon.
Providing effective management of loaned instrumentation processes and ensuring that those processes are standardized is critical. SP professionals must help establish and adhere to policies for all reusable surgical instruments that are not owned or consigned in their healthcare facility. One aspect of an effective policy is the time loaned instruments arrive in the SPD. To process loaned instrumentation thoroughly, loaned devices will ideally arrive at the user facility at least 24 hours ahead of the procedure (ideally, 48 hours in advance). This allows the SPD adequate time to properly receive, inspect, document, process and otherwise manage the loaned instruments. Note: For new sets not consigned to an organization, industry standards recommend that loaned instruments arrive 72 hours prior to the surgical encounter. This allows all parties involved with those instrument set(s) an opportunity to become familiar with them prior to use. For the SPD, special processing (disassembly, extended soak times, brushing, etc.) may be required. For the surgical team (technician, nurse, surgeon), it creates an opportunity to become familiarized with the instrumentation prior to the procedure.
Policy and procedure recommend that a loaned instrumentation program include (at minimum):
• Requesting loaned instrumentation or implant(s) when surgical procedure is scheduled
• Time requirements for pre-procedure processing (48 to 72 hours) and inservicing as needed
• Post-procedure processing and pickup (within 24 to 48 hours)
• Acquisition of loaned items, including a detailed inventory list (with photographs) and manufacturers’ detailed written IFU
• Cleaning, decontaminating, and sterilizing borrowed instrumentation performed by the receiving facility
• Transporting processed loaned instrumentation to the point of use
• Post-procedure decontamination, processing and inventorying
• Returning instrumentation to the representative of the company providing the loaned devices
• Maintaining chain of custody records for all transactions
Trust but Verify
Loaned instruments can be quite specific and complicated. It’s not uncommon for a procedure to require up to two dozen multi-layered instrument trays. Complicating matters further is that the SP technicians may be unfamiliar with the items, including what they are used for or how they should be arranged in the trays provided. Ideally, the manufacturer should provide a series of illustrations or photographs that align with the inventory list and corresponding product lot number. Those who validate and provide quality checks create robust processes that protect the organization financially. It is highly recommended that the SPD catalog and photograph every loaned item during receipt at the facility and release back to the vendor.
It is important for organizations to ensure that the loaned instruments they receive are complete and in good working order. Taking an inventory of the contents and cataloging each item keeps the process in check for both the vendor and the organization. Items in the set may be vital to the procedure; therefore, any missing, damaged or inoperable items could compromise patient care. Additionally, failure to inventory and document loaned items could needlessly cost the healthcare organization dearly if an item isn’t accounted for accurately.
Throughout this process, SP technicians assigned to intake (receipt) of loaned instruments should verify that the instruments are in good condition and free from discoloration, rust or pitting. If devices have been dipped or instrument taped, technicians must ensure that it has been applied correctly and is not damaged in any way. If an instrument is in poor condition, the issue must be documented clearly, and the vendor representative should replace it immediately.
Because loaned instrumentation may also travel frequently from one hospital to another, it is vital that the SP team inspects condition of the containers, baskets and trays, ensuring there are no dents, cracks or other signs of damage. It is essential to ensure that the lids fit securely, the seals are intact, and there is no rusting or sticky residue. Additionally, technicians must ensure that the locking mechanisms are functional, the valves or filter mechanisms are present and in working order, handles are intact and undamaged. Any issues identified should be reported to the company representative before accepting the loaned devices. Doing so will help prevent the facility from being billed for previous damage.
Power of labeling, weighing, communicating
Properly labeling and numbering trays with the requesting surgeon, procedure and patient allows the SPD and OR to keep track of loaned sets—keeping them together, organized and ready for use. If a procedure requires loaned sets from multiple manufactures, it will be necessary to label appropriately to ensure instruments are not mixed or combined.
All instrument sets, regardless of who owns them, should not exceed 25 pounds (this weight includes the container), according to ANSI/AAMI ST79. Heavier instrument sets may compromise the sterilization process, rendering instrument sets unsafe for use. In addition, sets exceeding 25 pounds may pose an ergonomic injury for employees.
For effective instrumentation management, open communication is always advised, and this is certainly important with loaned devices and when coordinating between multiple parties (e.g., surgeon, OR, vendor and SP) must occur. For example, in order for SPD to plan for the scope of work for which they will be responsible, it is important to know which loaned instrumentation is needed (the manufacture involved); the scheduled surgical procedure; patient; scheduled arrival time for the instrumentation; whether the instrumentation is consigned to the facility or will be arriving via a representative of the company or its courier; the number of instrument sets required for that particular procedure; and whether the manufacturer's IFU are available and accompanying the instruments’ arrival. Knowing this information in advance allows the SPD to plan accordingly. Planning may include but not be limited to increasing staffing to accommodate additional workload, training or in-servicing of staff (across all stakeholder departments) and ensuring IFU are provided and followed correctly.
Many factors must be considered when managing loaned medical devices, and thoughtful, comprehensive processes must be adopted and implemented to ensure timely use and safe, thorough processing. When all stakeholders agree to the policy, this prevents practice deviation and helps ensure that everyone involved has equal input and accountability.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019.
Additional Considerations:
The growing prevalence of loaned instrumentation in hospitals today can stem from:
• Insufficient inventory and/or lack of financial ability to purchase instrumentation
• Growing demand for surgical specialties (orthopedic, spine, etc.)
• Lack of storage space in healthcare organizations
• Clinical trials
• Patient-specific needs
• Scheduling conflicts and multiple procedures scheduled per day
• Owned instrument sets with missing or damaged items that are out for repair
Ineffective loaned instrument and implant management, including last-minute arrivals and the failure to obtain manufacturers’ instructions for use, can result in a host of problems for Sterile Processing and Operating Room personnel, including:
• A lack of processing time, which can lead to inadequate processing
• No IFU to guide the process (cleaning, decontamination, assembly, packaging, sterilization)
• Insufficient processing staff to manage workload
• Inability to process loaned sets due to the lack of training to work with complex medical devices
• Missing information to correctly label, identify or correlate with the patient or procedure
Sterile Processing Beyond the Operating Room
By Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST
This article originally appeared in the June 2023 issue of Healthcare Hygiene magazine.
The Healthcare Sterile Processing Association (HSPA) 2023 conference held in Nashville last month, had many recurring themes this year, but one seemed to stick out more than others -- Collaboration. When we see teams collaborating together, we find success, safety and prevention of infections and events. As attendees return to their busy roles and departments, it’s hard to remember the excitement and motivation felt when we were all in Nashville together, sharing ideas, listening to presenters, and learning together. If I can give one piece of advice post-conference, it is to stay connected with new friends, write down all of your new ideas and hopes from the presentations and conversations you had and never lose the excitement you felt after the conference.
In a session titled “Sterile Processing Beyond the Operating Room,” I took the audience on a journey through the hospital to all the areas that are not the actual operating room. What are these places? Where do you need to worry about having surgical instruments, scopes and other items that will be sent to sterile processing? The best way to find out is to go look! Some examples that all teams need to look at in their facility are nursing units, the Cath Lab, the EP Lab, Women’s Services, Interventional Radiology, Endoscopy, and the various clinics (ENT, Rad Onc, women’s clinics, etc.).
An example given was when the infection preventionist (IP) and sterile processing department (SPD) director when through every single nursing unit looking in the clean supply areas for sterile instrumentation. Not only will this give a chance to find sets that may be able to be retired (along with the surgeon who wanted that set to be created in the first place) but a chance to clean up the amount of one-time use and reprocessed instruments used in the ICU and medical units.
Second, perform an assessment in your clinical areas to see how instruments are being transferred to sterile processing; are the containers compliant with Association for the Advancement of Medical Instrumentation (AAMI) guidelines? Is there a pre-treatment spray being used? Do the team members know how to use the spray? Do they even know where the spray is? Many nursing teams have also learned that they will be asked questions by surveyors even if they are not using instruments on a regular basis on their clinical units—this is your chance to partner! Work on a routine schedule to provide education, in-services, fliers, etc., and a new partnership will be formed from this collaboration.
Through working with areas like Interventional Radiology and the Cardiology Cath Lab, it has become evident that there is a need for education on things like how to look for holes in a wrapped and reading the indicator on the inside of the tray—these are not topics of discussion as often as they may be in the operating room. Keeping track of compliance with transport and pre-treatment in these areas will be just as important, though lower volume than the OR, it will allow you to understand what the educational needs are.
A tip I learned many years ago as part of survey readiness was to always keep a list of all the scopes throughout the facility at any given time. This serves two purposes for the infection prevention and sterile processing team—you have a list you can use for auditing purposes, and you also have a list to provide any surveyor if asked when they come on site.
Working together, understanding the needs of our departments and teams and forming partnerships across the facility are all important aspects of collaborating for safety and success. HSPA 2023 in Nashville was a notable example of these partnership forming, collaboration happening and new ideas forming to move the sterile processing profession forward. The work we all do together, just like working together with departments and teams across our facilities, will keep our patients and team members safe in the future.
Jill E. Holdsworth, MS, CIC, FAPIC, NREMT, CRCST, is manager of the Infection Prevention Department at Emory University Hospital Midtown in Atlanta.
HROs and Just Culture: Core Concepts for SPD Quality
By Marie Brewer, CST, CRCT, CIS, CHL, CER, GTS, CLSSBB
This article originally appeared in the May 2023 issue of Healthcare Hygiene magazine.
Systems thinking, quality, patient safety, and high-reliability principles lead to sustainable process improvements because they help teams avoid serious safety events and continually improve patient and employee safety. Specifically, high reliability organization (HRO) concepts and “just culture” can serve as a strong basis for sterile processing (SP) programs and quality improvement and patient safety initiatives.
HROs effectively complete assignments in the face of high risk and complexity and use intricate procedures to manage their work and avoid failure or errors. HROs successfully handle near misses and address errors by implementing a mix of error-reduction methods that nurtures a safety-conscious environment.
Five key principles
HROs rely on the following five principles in daily operations, which lead to improved quality, safety and outcomes:
- Sensitive to operations: Understanding conditions in real time, regardless of intentions, designs or plans. This promotes a heightened awareness of how systems and processes are performing and encourages frontline staff members to report a situation in real time.
- Reluctant to simplify: Recognizing that work is multifaceted and refusing to dismiss or excuse failures without further investigation.
- Preoccupied with failure: Actively searching for potential errors and exploring all failures. HROs proactively work toward identifying possible failures and developing mitigation strategies.
- Deference to expertise: When patient safety is at risk, expertise is more important than the organizational hierarchy. HROs recognize that those who know the most about a subject should be entrusted to make the decisions.
- Commitment to resiliency: HROs value the ability to recognize concerns and create effective solutions. They react swiftly to a problem to mitigate its negative impact.
The foundation of an HRO lies in its philosophy of collective mindfulness—a non-judgmental consciousness of a group’s purpose, dynamics and mission. When a healthcare organization is collectively aware, the unified team works to collectively achieve their mission, vision and values. SP teams that practice mindfulness see improve team dynamics and increasing productivity and morale. The goal is for awareness and acceptance to replace reactivity and judgement. When applied to sterile processing departments (SPDs), HRO concepts encourage frontline technicians to look for and report concerns or unsafe situations before they present a significant hazard.
Accountability comes from the top; therefore, high-reliability concepts must be endorsed by the top leaders of the organization. A culture rooted in safety and process improvement is vital for supporting collective mindfulness and beneficial behaviors. It is essential that SP leaders reflect on their organization’s past and future endeavors and have a clear understanding of how the team promotes and supports HRO concepts. Leaders should ask the following:
- Do I round with employees directly? Rounding offers a direct line of sight to frontline staff concerns and allows time to share ideas with staff.
- How often do I review safety data, including the frequency of bioburden found on sterilized devices, missing indicators, etc.? Safety reviews help reinforce the team’s commitment to resiliency.
- Do I lead and participate in safety huddles to share concerns and communicate error mitigation strategies? Doing so helps ensure that the team defers to expertise and remains focused on preventing errors proactively.
HROs emphasize the need to follow standard operating procedures (SOPs), written instructions that detail a step-by-step process that must be followed to ensure a routine activity is performed properly and consistently, even during times of high stress. HROs make fewer mistakes; however, when errors do occur, critical information is disseminated to alert staff to the mistake and allow the team to fix the error(s) more effectively efficiently. Further, these organizations learn from mistakes by instituting just culture, which discourages penalties for reporting an honest mistake. What’s more, HROs rely on strong communication and problem-solving skills and realize that technical expertise alone is not enough. SP leaders must be able to communicate in a manner that earns trust and support.
Just culture promotes safety, collaboration, education, communication and teamwork, which means mistakes can be viewed as learning opportunities. All team members should be involved in the process and participate in after-action reviews (AARs); these are used to debrief a project or event to determine what occurred and why and how to improve it. Leaders should encourage improvement and idea sharing and avoid taking a punitive approach when mistakes occur. HRO managers design common goals and create social pressures to adhere to SOPs. Every individual should understand how their performance affects the team, and leaders should perform routine readiness reviews (RRRs) and explain how each member’s role affects the mission.
When a mistake is reported or identified, is helpful for the SP leader to pause and breathe before reacting. Being a calm and approachable leader who fosters a culture of psychological safety allows employees to feel more comfortable admitting their mistakes and encourages a team-based approach where SP technicians and leaders work together to identify ways to prevent recurrence.
Systems can fail and complex systems often fail in complex ways. Therefore, process improvement action planning should be robust, viable and sustainable. Effective action planning allows team members to do the right thing, even when it is difficult, and can be facilitated by adopting technology to increase efficiency and capture process-specific data; adding another technician to verify low-volume, high-risk procedures; and adding appropriate administrative and engineering controls. To make change sustainable, the reason behind the change must be explained to all employees, so its purpose is not questioned, and new processes are always followed properly.
HROs remain focused on long-term performance improvement across all stages of their work. They strive to remain resilient in their processes, even during disasters and other high-pressure events. These quality-focused organizations are also able to decrease mistakes by following SOPs, participating in AARs and increasing employee accountability in positive, non-punitive ways. The adoption of just culture and a thoughtful approach to quality and safety creates a departmental culture that is rooted in teamwork and accountability. The result is a shared understanding that all SP employees play a vital and equal role in quality service and positive patient outcomes.
Marie Brewer, CST, CRCT, CIS, CHL, CER, GTS, CLSSBB, serves as sterile processing manager at UnityPoint Health–St Luke’s Hospital, Cedar Rapids, Iowa.
SPD-Related Lawsuits: Who’s to Blame and How to Cut the Risks
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the April 2023 issue of Healthcare Hygiene magazine.
Recently, a lawsuit was filed against another prominent healthcare organization after patients suffered postoperative complications due to contaminated instruments. The organization’s executives, as well as some leaders from other facilities that faced similar legal action, have been quick to admit sterile processing-related process gaps; however, it is essential to recognize that the blame should not be shouldered solely by sterile processing (SP) professionals.
As a healthcare consultant who assists dozens of hospitals across the U.S. each year, these so-called SP-related process gaps are often due to system-wide organizational failure. SP professionals across the country are asked to perform many challenging tasks with too few resources and inadequate support. Limited SP staff and instrumentation inventories, inadequate training and outdated equipment are all too common. I’ve seen so many SPDs that are woefully undersized and understaffed and have insufficient (outdated and malfunctioning) equipment, supplies and training that it’s little wonder negative processing outcomes occur.
One SPD I visited recently was so cramped, dated and underserved that the technicians prepared and stored case carts in unkempt, unrestricted basement hallway. Another I visited did not have enough sinks, failed to use the ultrasonic, and for six months had two broken spray arms in the washer-disinfector. When I presented the concerns to senior leadership, they admitted they were aware, but lacked the budget to replace or repair the equipment. What’s more, I’ve seen countless instances where SP technicians aren’t provided the latest standards and instructions for use (IFU), so, they are unaware of the correct processing procedures and best practices.
Equally troubling is that many facility executives and SPD healthcare customers do not understand the many vital processes and practices that must occur in the SPD to ensure successful processing outcomes—and how critical it is that end users manage devices safely and effectively during and after the procedures to assist processing efforts.
Although the SPD, OR and other procedural areas have different functions, they share the same goal: providing the safest, highest-quality patient care. Still, each department bears complex responsibilities and faces different challenges and expectations that can strain interdisciplinary relationships and, at times, lead to finger pointing when device-related incidents arise. It is vital that those from the SPD and user areas work together to educate about requests, needs and concerns, and partner to ensure everyone understands their role in effective handling, treatment and processing of reusable medical devices.
To uncover root causes of device contamination and resulting infections or other related complications, healthcare organizations should perform a multi-departmental process assessments to identify contributing factors for the incident(s). Many critical steps must be taken before instrumentation or other reusable devices can be re-sterilized or high-level disinfected, some of which are performed at the point of use.
Point-of-use treatment is an essential preliminary steps to effective instrument processing; spraying devices with moistening agents or enzymatic solutions whenever appropriate (or keeping instruments moist by covering them with a sterile–water-moistened towel) prevents bioburden and other material from drying and hardening on devices and making decontamination more difficult. This critical step is, unfortunately, often rushed or skipped altogether, making decontamination far more difficult and potentially impeding the sterilization process.
Device mishandling is another common occurrence by end users in procedural areas. Instruments may be misused, which can lead to breakage or patient injury; instrument sets may be carelessly mixed, increasing the likelihood for lost or misplaced devices; heavy instruments may be incorrectly placed atop delicate devices; cords may be too-tightly coiled; and more—all of which can contribute to costly damage and negative patient outcomes.
It is also helpful for SP leaders and physicians to work together to optimize instrument sets, remove duplicate or frequently unused devices from sets, and ensure that clinicians are educated on and comply with current standards, IFU and facility policies and procedures. This means ensuring they handle instruments and equipment with care, promptly returning post-procedure devices to the decontamination area and allowing enough time for instruments to be thoroughly processed for the next procedure.
Patients place their trust and lives in the hands of their healthcare providers. Infections and other negative patient outcomes can arise because of inadequate or incorrect practices, such as those related to instrument and equipment care and handling and processing, but the problem doesn’t rest solely on those in the SPD. To mitigate those risks, leaders must encourage interdisciplinary partnerships and proactively assess practices in the SPD and procedural areas to ensure each department has the training, resources and support to perform their duties safely, consistently and effectively.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC in San Antonio, Texas. He has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019.
Prioritizing SPD Service Excellence Spurs Safety, Positive Outcomes
By Tony Thurmond, CRCST, CIS, CHL, FCS
This article originally appeared in the March 2023 issue of Healthcare Hygiene magazine.
In the absence of knowledgeable, skilled and safety-focused sterile processing (SP) professionals, Surgical services staff and those in other patient-care areas the sterile processing department (SPD) serves would not have the required instrumentation and equipment to perform surgical and medical procedures. What some SP professionals may not realize, however, is that the quality of the work they provide every day not only hinges on job competencies and standards knowledge but also the way they interact with their departmental and interdisciplinary teammates.
All healthcare workers, including those in the SPD, must consider what makes their encounters with others in the organization successful and productive. It is rewarding to provide services for a customer (and ultimately, the patient), but we can also benefit by reflecting on customer service and our ongoing exchanges with those we serve and with whom we work. It’s not uncommon to complete a task or service request and then move to the next one with little thought, especially during more demanding, hectic shifts (or when service is delivered without disagreement, discontentment or other challenges). However, becoming more aware of our customer and co-worker interactions and more intentional with how we respond during those interactions will strengthen relationships and prompt even greater communication and service delivery.
Service-oriented individuals anticipate and strive to appropriately meet the needs of co-workers, customers and others within the organization. They demonstrate integrity and compassion for those they serve and recognize that each interaction and instrument processed should be done in a manner that contributes to the safest, more effective and efficient patient care possible. SP teams committed to meeting the needs of all departments they serve within their organizations must exhibit some crucial traits to help them provide consistent, high-quality service, even when faced with seemingly insurmountable pressures and challenges. When practiced and honed, these traits and characteristics will contribute to improved service and positive patient outcomes.
Empathy: Empathy is the ability to understand another person’s view, feelings or experiences. While another individual’s perspective may not always align with our own, we should work to understand another’s perspective. Those who work (and lead) with empathy gain more understanding of customers’ needs, allowing them to respond to service requests more effectively and expeditiously. If an OR circulator requests an item by an incorrect name, for example, SP technicians should recognize that circulators do not routinely scrub for procedures and may not know the correct name of the device needed—and the item could be urgently needed. Understanding that the circulator’s current environment may be tense and uncomfortable and that their tone or mannerisms may reflect that situation will help those in the SPD to respond to the request calmly, helpfully and efficiently.
Adaptability: SP professionals’ ability and willingness to shift, remain flexible and learn new ways of meeting customer needs is crucial. Each SP customer differs in their needs and the urgency of their requests, and service-oriented SP professionals are prepared to adjust their approaches and communication with each encounter. SP professionals (technicians and leaders) must be able to readily adapt to handle new expectations, demands and challenges.
Communicating effectively: Communicating clearly and precisely with customer and co-workers is vital for effective, productive interactions and outcomes. Service requests should be viewed as opportunities, not interruptions, and SP professionals must convey information clearly and promptly. Those in the OR and other service areas where urgent care is provided may not always understand why instruments or equipment can’t be provided immediately or as expeditiously as they may like (following the procedure or at another less-hectic time, SP professionals can benefit by sharing with customers the latest standards and instructions for use and why they each step in the process must never be skipped or rushed). It is vital that SP professionals clearly explain all available options, while also sharing as accurately as possible how long it will take to fully process and deliver an instrument or set for patient care. Following up to confirm that the SPD effectively met the customer department’s needs (and working collaboratively to improve service if errors occurred) also strengthens interdisciplinary communication).
Trustworthiness: Being a collaborative, safety-focused, transparent problem-solver who aligns with the latest standards, guidelines and best practices should be every SP professional’s primary goal. Mistakes happen and challenges and conflicts arise, but all healthcare professionals must be honest and forthcoming when errors and obstacles occur so proper resolutions can be collaboratively sought. No person knows every answer, but those who seek to find effective and timely solutions will be viewed as reliable resources and trusted teammates.
Knowledgeable (without limits): Engaging in continuing education and less-structured knowledge-building opportunities advances professional growth, boosts personal confidence and helps SP professionals stay sharp and navigate new challenges. New knowledge is cultivated by positive and difficult experiences and outcomes, allowing staff members to learn from mistakes and difficult scenarios. SP professionals can also benefit themselves, their co-workers and customers by deepening their understanding of others’ roles, needs and responsibilities. Familiarizing oneself with less-common or more challenging medical and surgical procedures, for example, can help SP technicians broaden their instrumentation knowledge and respond more effectively to unique or urgent requests.
SP professionals’ commitment to delivering the best possible service and communication with customers and co-workers strengthens teams, enhances collaboration and builds better relationships. With practice and intention, each of these traits can be sharpened, service delivery will be improved, and better patient outcomes will result.
Tony Thurmond, CRCST, CIS, CHL, FCS, serves as central service manager for Dayton Children’s Hospital. He is a past-president of the Healthcare Sterile Processing Association (HSPA) and a current HSPA board member.
Service-Oriented Sterile Processing Professionals: What It Means for Positive Outcomes
By Tony Thurmond, CRCST, CIS, CHL, FCS
This article originally appeared in the February 2023 issue of Healthcare Hygiene magazine.
In the absence of quality-focused, dedicated and skilled sterile processing (SP) professionals, operating room (OR) staff and other patient care departments would not have the instrumentation and equipment required for patient care. Meeting those needs requires SP professionals to remain dedicated to their competencies and ongoing knowledge advancement in the name of service excellence—and also effective communication with their healthcare customers to ensure needs are met and challenges are proactively addressed.
What makes for successful customer support and interaction? It is satisfying to complete a task for our customers, but it’s also essential to reflect on the encounter to assess opportunities for improvement. Unfortunately, with hectic and harried schedules, many may move on quickly from a conversation or other interaction, especially if the encounter was a positive one. But every exchange presents opportunities for growth and improvement. We just need to seek those opportunities.
Being service oriented is a helpful activity to supplies aid as needed and leads individuals to anticipate challenges and future needs and recognize how to best serve our co-workers and customers. A service-oriented person cares about others and strives to be available to meet their needs, efficiently, effectively and empathetically. Put simply, they are committed to enhancing the service they provide, even when that service is considered exemplary by healthcare customers. There’s always room for improvement, especially in the healthcare segment when patients’ well-being lies in the balance.
To develop ourselves as stronger service-oriented SP professionals, it is helpful to consider our everyday interactions. We interact with Surgical staff daily, for example, and they often look to us for answers and solutions to challenges the arise. This approach is often rooted in need and demand, not necessarily one that promotes effective interdisciplinary exchange or improved service. SP teams require tools and support to help them provide exemplary service in the face of demanding and challenging situations. The tools—knowledge, empathy, adaptability, communication and trustworthiness—may seem simple to attain, but they take effort and practice. Let’s explore how each tool can positively impact SP service quality.
Knowledge: Continued efforts to build job knowledge helps guide SP professionals through situations that may at first seem impossible to overcome. New knowledge often comes from experience, both positive and negative. Learning new roles and responsibilities helps all professionals gain that knowledge and also more confidence in their day-to-day roles and customer encounters. For example, it is helpful for SP professionals to study unfamiliar procedures, brainstorm scenarios where their help might be most beneficial, networking with other professionals to share experiences and identify best practices, and then sharing that information with others, including teammates and customers. Knowledge is valuable but only when it is put to good use and shared.
Many of us focus on the OR and other procedural areas as our customers, but we should also consider our own departmental co-workers as customers and adopt ways to support them more effectively. Our departmental teammates benefit greatly from our help, guidance, knowledge, mentorship and solid communication skills.
Empathy: Empathy is the ability to understand another person’s view, feelings or experiences. While their perspectives will not always align with our own, we have the responsibility to attempt to understand one another’s stance and seek common ground. A person who leads with empathy better understands each customer’s role and environment and respond appropriately. For example, when an OR circulator calls the SPD and asks for an instrument by the incorrect name, it’s important to remember that circulators are often mere messengers who often do not scrub for procedures and may not know the exact item they are requesting. The circulator may sometimes call with an urgent request due to an incident arising during a critical point in the procedure. Understanding that the circulator’s (or other OR professional’s) current environment may be tense and that their tone and urgency is rooted in a high-pressure circumstance can go a long way toward servitude and improved understanding. Having empathy and compassion in such situations helps SP professionals navigate a request more calmly and efficiently.
Adaptability: Nearly every job description in the SPD either requires flexibility and an ability to adapt to various pressures and challenging circumstances. The ability and, more importantly, the willingness of SP professionals to shift, adapt and learn new ways of working with customers is necessary in today’s fast-paced, high-pressure environment. Each SP customer is different and has a variety of needs, so SP professionals must be prepared to adjust their approach with each interaction.
Communication: Communicating clearly and precisely is crucial for every customer interaction. Avoid approaching a service opportunity with the mindset that the request is an interruption or inconvenience, and work at bringing a solution to a problem. Explain the available options and maintain a pleasant, helpful tone. Equally important is following up with the customer to confirm that the service provided met their need. If not, openly discuss what would have been a better alternative.
Trustworthiness: Being a solutions provider for customers must be a top priority for all SP professionals. They will not always have an immediate answer to every situation, but they should commit to seeking answers in the name of service excellence, knowledge growth and professionalism. Showing consistent initiative to find the best solutions to problems in a timely manner builds interdisciplinary trust and more powerful relationships.
SP professionals’ willingness to continually improve their support for co-workers and external customers helps build a stronger, more unified team that stays focused on best practices and service excellence. Providing the very best service not only helps create more satisfied SP professionals but also fosters greater satisfaction from healthcare customers, while promoting the highest level of care for patients—the most important customer of all.
Tony Thurmond, CRCST, CIS, CHL, FCS, serves as central service manager for Dayton Children’s Hospital. He is an HSPA Past-President who currently serves as a director on the Healthcare Sterile Processing Association (HSPA)’s board of directors.
Key Updates to AORN’s Revised Flexible Endoscope Processing Guideline
By Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS
This article originally appeared in the January 2023 issue of Healthcare Hygiene magazine.
The Association of periOperative Registered Nurses (AORN) recently released its updated Guideline for Processing Flexible Endoscopes, which incorporates research-based recommendations on flexible endoscope processing. The revised document provides guidance across all stages of flexible endoscope processing, beginning with point-of-use treatment and followed by leak testing, manual cleaning, cleaning verification (CV), inspection, sterilization or high-level disinfection, drying, transport and storage. Further, the updated guideline provides recommendations for pre-purchase evaluation of reusable and single-use flexible endoscopes, the endoscope processing environment and related processing equipment.
Significant additions also made it into the revised guideline, beginning with the preliminary step of pre-purchase evaluation of flexible endoscopes, equipment or supplies that will be used for processing. Before any such items are purchased, AORN recommends gathering an interdisciplinary team to create a standardized process for product evaluation and selection. This team should include sterile processing (SP) professionals, and the product evaluation and review process should include examining criteria for flexible endoscopes, automated endoscope reprocessors (AERs), storage cabinets, borescopes, cleaning solutions, and detergents.
Sterilization and HLD updates
Similar to ANSI/AAMI ST91:2021 Flexible and semi-rigid endoscope processing in health care facilities, the updated AORN guideline recommends sterilizing reusable flexible endoscopes that have a manufacturer validation for sterilization whenever possible. The recommendation is consistent with the Spaulding Classification system, which labels items, such as flexible endoscopes, that come in contact with non-intact skin or mucous membranes as semi-critical devices and are recommended to be processed by sterilization or, at a minimum, by HLD. Additionally, flexible endoscopes and accessories that enter sterile tissue should be sterile when used or placed on a sterile field.
Not all flexible endoscopes can undergo sterilization according to their manufacturer’s instructions for use (IFU). Still, the recommendation notes that sterilization provides the highest level of assurance that processed items are free of viable microbes. If a liquid chemical sterilization (LCS) system will be used to sterilize an endoscope for a critical procedure, the endoscope should be immediately transported to the point of use in a closed processing container. Endoscopes used for a semi-critical procedure and are processed in a LCS system may be processed in the same manner as those that receive HLD.
Conversely, if processing flexible endoscopes using an HLD method, it is recommended to use a compatible Food and Drug Administration (FDA)-cleared AER per the manufacturer’s IFU. This process includes verifying that all connections are correct and then monitoring the cycle using all recommended monitoring tools. If manual HLD is performed, it is recommended to process endoscopes with a compatible FDA-approved high-level disinfectant in accordance with the manufacturer’s IFU and the AORN Guideline for Manual Chemical High-Level Disinfection. It is recommended to use an automated process for HLD instead of a manual process; automated processes may be more efficient and consistent and reduce employee exposure to high-level disinfectants. It is also recommended that flexible endoscopes be reprocessed the same way across all processing locations and shifts, including weekends.
Point-of-use treatment and leak testing updates
The point-of-use treatment recommendation now includes more point-of-use treatment steps. It includes a high-level diagram of a basic flexible endoscope and diagrams of three different types of flexible endoscope distal ends that emphasize their complex designs and differences.
Recommendations for endoscope transport to the decontamination area now include a hand-over process from the transporter to decontamination personnel. The process includes a list of information to accompany the endoscope; this should include the time that point-of-use treatment was completed, whether that treatment began immediately after the endoscope’s use, and whether the endoscope was kept moist until point-of-use treatment was performed. Another important point communicated in the guideline is what the endoscope was exposed to during the procedure (e.g., simethicone, lubricants, tissue adhesives).
Leak testing is an especially vital step in flexible endoscope processing. Verification of the leak tester’s pressure accuracy or calibration of automatic leak testers ensures that the leak tester is producing the correct pressure. Under-pressurizing an endoscope may allow a leak to go undiscovered, and over-pressurizing may stress the seals and damage the endoscope. A new recommendation was added to verify the pressure of the leak tester in accordance with the manufacturer’s IFU. When using an AER that has a mechanical leak test, it is also recommended that the leak test performed by the AER be considered as an adjunct to leak testing performed before manual cleaning. Note: Mechanical leak testing in an AER is not a substitute for leak testing performed before manual cleaning as recommended in the endoscope manufacturer’s IFU.
CV, drying, storage & transport
New recommendations for cleaning verification (CV) were added. The previous version of the guideline featured a conditional recommendation that internal channels may be inspected with a borescope, whereas the new version recommends using a clean borescope to visually inspect accessible channels of flexible endoscopes before sterilization or HLD. Healthcare organizations should identify high-risk flexible endoscopes and those devices should undergo CV testing after each use, with the results documented. If the endoscope does not pass the CV test, the endoscope should be re-cleaned and re-tested. If the device repeatedly fails CV testing, it should be removed from service and labeled as needing repair to prevent it from being used.
Research has shown that moisture retained in processed flexible endoscopes has been associated with patient infections, biofilm growth, microbial contamination and increased adenosine triphosphate (ATP) values. Thorough drying is so essential that there is a recommendation to perform drying even when using an AER with an air purge cycle or extended dry time feature. It is recommended to dry all accessible channels for at least 10 minutes and, if visible moisture is still present, to extend the dry time until visible moisture is no longer observed. The recommendation also provides guidance on using the endoscope immediately or placing it into storage. Storage time for endoscopes varies for each facility based on numerous factors. To help determine endoscope storage time, a new conditional recommendation was added on conducting a storage time risk assessment.
A new section was added on transporting the endoscope to the point of use. Recommendations are provided for how to transport endoscopes, which type of container to use, and how to properly clean and disinfect the container. A conditional recommendation is provided that does not require an endoscope to be placed inside a transport container when transporting it to the point of use as long as it is transported through a controlled and connected corridor within the Endoscopy suite.
Another section was added on prion transmission (e.g., Creutzfeldt-Jakob Disease). Evidence shows that flexible endoscopes may be used on patients who are at an increased risk for prion diseases as long as the devices are processed using standard procedures for cleaning and sterilization or HLD. The reason for this recommendation is that flexible endoscopes do not become contaminated with the high-risk tissue for prion transmission and, therefore, do not present a risk for prion transmission.
Education and competency recommendations
The revised guideline’s leadership recommendation states that leaders should ensure there is sufficient time and staffing to perform all processing steps in accordance with the manufacturer’s IFU, standards and regulations, and recommendations are provided to help leaders achieve those critical goals. With research showing the importance of point-of-use treatment, the revised version also features education-based recommendations for personnel at the point of use who handle flexible endoscopes.
Further, while the use of borescopes allows processing personnel to detect debris and other flaws within the channels of endoscopes that may otherwise go undetected, these inspection devices may present challenges regarding identification and interpretation of findings. In light of that, a recommendation was added for personnel who perform borescope inspection of accessible flexible endoscope channels before sterilization or HLD to receive education and complete competency verification on borescope activities.
This article provides only a high-level summary of some of the key changes to the revised AORN Guideline for Processing Flexible Endoscopes. The complete revised AORN Guideline for Processing Flexible Endoscopes provides more information and has been updated in the eGuidelines Plus at https://www.aorn.org/eguidelinesplus. The 2023 print version is available for purchase on the AORN website at www.aorn.org/.
Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS, is a clinical educator for the Healthcare Sterile Processing Association (HSPA).
Enhanced SPD Cleaning Practices Aid Infection Control Efforts
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the December 2022 issue of Healthcare Hygiene magazine.
Preserving our commitment to patients requires significant moral courage and years of exceptional preparation. Today’s sterile processing (SP) technicians practice in very complex, stressful environments, which can lead to missed steps or complacency and create the potential for a high-risk environment that can jeopardy employee and patient safety. SP professionals are exposed to potentially deadly bloodborne pathogens, healthcare-acquired infections (HAIs), and a host of other environmental exposures. Operational factors and clinical characteristics in sterile processing departments (SPDs) can influence outcomes, including the risk for surgical site infections (SSIs) and an increase in morbidity and mortality rates.
Cleanliness is imperative in any healthcare setting, and in the SPD, that pertains to instruments, equipment and the environment itself. Unfortunately, nosocomial infections continue to harm patients. SSIs alone continue to affect 2 percent to 5 percent of surgical patients.1 It is estimated that 40 to 50 million surgical procedures are performed annually in the U.S.2 Statistically that totals 800,000 to 2.5 million SSIs per year, which cost U.S. hospitals an estimated $3.3 billion, extend hospital lengths of stay by 9.7 days, and increase the cost of hospitalization by more than $20,000 per admission.3 How many of those infections are attributed to SPD is unknown; however, failing processes, missed steps or poorly managed departments can be a factor.
SPD cleaning often falls short
In the second half of the 19th century, Florence Nightingale’s developed the environmental theory focusing on external conditions that suppressed or contributed to disease or death. Using mathematical statistics to prove cleanliness made a difference; she found that soldiers were 10 times more likely to die from “filth diseases,” such as cholera, dysentery, typhoid and typhus, than those who were injured by bullets or cannon fodder.4-5
Although environmental services (EVS) professionals are responsible for more thorough departmental cleaning, it is still the role of every SP profession to ensure that their workspaces are kept clean, and orderly and that trash and other debris are picked up from floors and other surfaces, and properly disposed. Unfortunately, in my consultant role, I’ve seen countless instances where SPDs and other areas where instrument reprocessing occur are not being cleaned and maintained properly. In fact, when working with numerous clients throughout the country over the past several years, I have rarely seen SP staff damp-dust their workstations or even restock their supplies as they should. I’ve also witnessed sticky substances from tape and chemicals are often found on various surfaces (workstations, instrument pans and baskets, computers, and even on instruments) and unclean and clutter workspaces are all too common—and many facilities fail to notice.
Work areas (decontamination, assembly/sterilization and storage areas) must be cleaned daily, prior to the start of each shift. Individual workstations should be cleaned daily and as needed. Sticky residues, tape and other substances must be removed prior to cleaning devices and other surfaces to ensure the appropriate chemicals used for cleaning come in contact with the surface. Equipment should also be wiped down daily, and areas behind and around processing equipment should be free from dust, dirt, dead insects, wrappers and other debris.
Budgets’ impact on cleanliness
Hospital procedural areas are revenue generators. In some cases, surgical services can generate two-thirds of topline revenues and as much as 60 percent of an organizations margin.6 As reimbursement opportunities continue to erode, hospitals are faced with making difficult decisions to maintain their budget, and non-revenue-generating departments, such as the SPD, may face the most budgetary restrictions. If budgets affect hiring, for example, there may be too few technicians in the department to keep up with the instrumentation volume and also properly and consistently maintain the departments. It’s a slippery slope that can negatively affect employee and customer satisfaction, while also increasing the risk for contaminated surfaces and devices.
Daily, weekly and monthly cleaning should be supported by shift leaders. They should ensure employees across all shifts understand the cleaning requirements and why they are so important to infection prevention and patient and staff safety. Documenting routine cleaning is important and SP supervisors and managers should consistently review cleaning logs to ensure they are being maintained. Terminal or deep cleaning of walls, ceilings, light fixtures, shelving units, underneath and behind equipment and so on occur on a regular schedule (weekly, monthly, quarterly or as needed/determined by the facility). If areas or surfaces in the department remain unclean after EVS treatment, the SP manager should consider taking photographs of the issues and sharing them with the EVS manager.
In conclusion, maintaining clean, well-organized departments is essential for operational efficiency and productivity, but more important, for helping prevent infections. SP leaders must ensure that their teams have the training, resources and support they need to keep their department and work areas clean and tidy. Doing so will help promote employee satisfaction, while also improving patient care and positive outcomes.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as an HSPA contributing author since 2019.
References:
- Nosocomial infections: Epidemiology, prevention, control and surveillance. https://ac.els-cdn.com/S2221169116309509/1-s2.0-S2221169116309509-main.pdf?_tid=6aa11690-0dd8-11e8-90f1-00000aab0f27&acdnat=1518208559_38b0d1a3414db0fa63e9bf467593db09
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7388795/#:~:text=Globally%2C%20a%20staggering%20310%20million,and%2020%20million%20in%20Europe.
- https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- The Florence Nightingale’s Environmental Theory: A Critical Analysis http://www.scielo.br/scielo.php?pid=S1414-81452015000300518&script=sci_arttext&tlng=en
- How Florence Nightingale cleaned up “hell on earth” hospitals and became an international hero. https://www.pbs.org/newshour/health/florence-nightingale-cleaned-hell-earth-hospitals-became-international-hero
- Perioperative Leadership: Managing Change with Insights, Priorities, and Tools, AORN Journal, July 2014 Vol 100 No 1
For further reading:
- Central Service Leadership Manual, Third Ed. “Maintaining a Clean Environment.” pp. 141–142. HSPA. 2020.
- Central Service Technical Manual, Eighth Ed. “Work Area Cleanliness.” pp.113–114. HSPA. 2016.
Honorary Weeks for SP, IP, OR Disciplines Offer Unique Ways to Strengthen Teamwork
By Julie E. Williamson
This article originally appeared in the November 2022 issue of Healthcare Hygiene magazine.
Sterile Processing Week and International Infection Prevention Week were both celebrated a day apart in October, and November brings Perioperative Nurse Week (Nov. 13–19). All three consecutive events create a perfect opportunity to improve interdisciplinary support, appreciation and recognition.
The Healthcare Sterile Processing Association (HSPA) encourages sterile processing (SP) professionals to extend their reach beyond their department walls and strive to bridge gaps and strengthen partnerships with operating room (OR) professionals, infection preventionists and other interdisciplinary teammates. The OR relies heavily on SP professionals to provide clean, sterile, well-functioning, and properly maintained and managed instruments and equipment to meet patient and procedural needs throughout the day. Each SP professional’s dedication to following the latest industry standards, guidelines, best practices, and instructions for use (IFU) directly affect infection prevention and control efforts, quality customer service and patient outcomes. At the same time, OR team members can contribute to the success of their SP partners by performing proper point-of-use treatment on instruments during and immediately following their use and then ensuring all used devices are transported as quickly as possible to the SPD’s decontamination area to help prevent bioburden from drying and hardening on instruments and difficult-to-remove biofilm from developing on their surfaces.
Although SP and OR professionals—and even infection preventionists (IPs)—have different core roles, each shares an essential mission: preventing infections and promoting safe, timely, high-quality patient care. The close interworking of these three allied professions accentuates the value of taking time to honor each during their official celebratory weeks and then fostering strong and productive teamwork throughout the year.
Amp up the appreciation, education
In October, HSPA and Healthcare Hygiene magazine partnered for the 2022 virtual Symposium on Sterile Processing & Infection Prevention, which provided free top-quality education (worth six continuing education credits) and valuable networking opportunities. The three-day live event launched during Sterile Processing Week (Oct. 11-13), with all content now provided on-demand for registered participants through Dec. 31, 2022. Some of the event’s education addressed the critical importance of aligning SP professionals with their IP and OR peers and other allied departments, and even fostering more effective relationships with those in the C-suite. (If you missed any of the sessions, they are now also accessible on the Healthcare Hygiene magazine website at: https://www.healthcarehygienemagazine.com/test-2/sp-symposium-sessions/)
During each discipline’s honorary week, it is beneficial to invite broader participation and support through departmental tours, targeted educational offerings and open house events. For Sterile Processing Week, for example, HSPA encourages SP managers to invite other departments and facility executives to the department for a tour of each SP area, educational in-services, potlucks, and other activities promoted by the SP team that can help demonstrate the profession’s many vital roles and contributions.
“When people who aren’t overly familiar with the SPD come to the department, they are often shocked by all that takes place there, and how much detail and expertise is required to complete every step and stage in the process,” said HSPA director of education Natalie Lind, CRCST, CHL, FCS. “Including customers, executives and others to the SPD helps promote valuable teaching and communication moments. The OR and other direct patient care areas the SPD serves can receive valuable hands-on training about proper point-of-care treatment and prompt delivery of used instruments to the SPD, effective management of loaned instruments, biofilm risks, standards updates, facility policies, avoidable instrument damage, and more.
With Infection Prevention Week immediately following Sterile Processing Week, and Perioperative Nurse Week approaching shortly thereafter, reciprocal arrangements are a prudent pursuit. As Lind points out, SP managers can invite their IPs and healthcare customers to present their own brief education during Sterile Processing Week, and then those departments can invite their SP teammates to their events to learn more about how the devices processed in the SPD are used in patient care. OR nurses, for example, can speak directly with SP staff during Perioperative Nurse Week to educate about particular instrumentation-related issues that challenge the OR team.
Of course, there are many other ways to recognize interdisciplinary partners and patient safety allies during their honorary weeks and throughout the full year. Each department can consider giving heartfelt cards and letters of appreciation—all signed by departmental teammates, across all shifts. [Note: HSPA created free, downloadable and customizable Sterile Processing Week templates, including certificates, awards, thank-you cards, and wall posters that can serve as templates for yearlong recognition and even be tailored to acknowledge the SPD’s support of IPs and OR nurses, for example. Visit https://myhspa.org/about/sp-week.html to access the downloadable items.]
“Thoughtful notes with a plate of baked good or other seemingly small tokens of appreciation can be very meaningful and help make all of our dedicated professional weeks of honor even more special and memorable,” Lind added, noting that not only should managers and supervisors take time to recognize their staff members, but they should also encourage their employees to recognize their peers. “People do good things every day, all year, so we should draw attention to that frequently. If we see someone going above and beyond, let’s recognize that and support their efforts. This is true of our own internal teams as well as those in the departments we serve.” Indeed, it does not take much to say, Thank you for your help or Thank you for your patience and understanding, or We appreciate the work you do and your willingness to partner even stronger for patient safety, Lind continued.
“This type of positive reinforcement and recognition, provided verbally and in writing, is what builds long-lasting camaraderie, promotes effective knowledge sharing and facilitates more effective teamwork. All that plays a significant role in quality service, patient safety and other positive outcomes.”
Julie E. Williamson is director of communications for the Healthcare Sterile Processing Association (HSPA).
Workplace Wellbeing: Overhauling Sterile Processing Employee Engagement Strategies
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the October 2022 issue of Healthcare Hygiene magazine.
Employee engagement and satisfaction are critical components of any organization’s success and necessary ingredients for retaining and attracting top talent. A survey conducted by the American Psychological Association (APA) found that feeling valued at work is directly linked to better physical and mental health, higher levels of engagement, satisfaction and motivation.1 For that reason, today’s healthcare leaders must work to develop strategies that will inspire and empower their staff members to deliver their very best work every day and on every shift.
When an employee feels valued, it can significantly improve their workplace experience, leading to a healthy and productive relationship between the organization and employees. In turn, this directly benefits the company through improved productivity, efficiency, and staff retention.
Lead by example
Sterile processing (SP) leaders should lead by example and bring the same level of passion and energy to their roles as they expect from their employees. Showing employees what is expected of them always carries more weight than simply telling them what to do. When a leader is willing to pitch in and perform daily tasks of staff members to help ease their burdens, employees tend to respect those leaders more. Engaging employees is as much about giving them meaningful work as it is about creating an environment where they experience engagement daily.
If leaders are not passionate about their employees or are not adequately engaged in their own role, it’s going to be hard for anyone reporting to them to remain engaged. Author’s note: In my leadership positions, I never want to see my employees waiting for something do you. When they respond with, “Everything is done,” after being asked if more work must be done, that is my cue to engage them in other areas. I don scrubs and PPE and those staff members who are caught up with their tasks tour the department with me. During these tours, it’s not uncommon to identify disorganized or outdated supplies, tape or sticky residue on walls and equipment, or dusty and dirty areas that require attention. At that point, I start assigning the employees to various tasks and I share with them my philosophy: “If you have time to lean, you have time to clean.” It is important to note that I also tackle some of those tasks and clean and organize alongside them. This gives me an opportunity to remember what it is like to be a technician and understand their roles and challenges. When the tasks are complete, I share with them that a clean and organized department is an expectation and, going forward, they must manage that expectation. When employees know their clear expectations, they can rise to meet them and take pride in the work they do.
Create a culture of gratitude, respect
SP leaders and other organizational leadership and executives who show gratitude for the work employees perform daily can significantly improve staff members’ workplace. Gratitude shared among employees also pays big dividends. Leaders who develop peer-to-peer recognition platforms can make recognition more authentic and deeply focused on individual qualities and contributions. When praise is given to employees, satisfaction can improve, and new opportunities can emerge.
One effective strategy I recently discovered and added to my engagement initiatives was to encourage staff to recognize one other with notes and words of wisdom and appreciation. This public display of appreciation can build confidence, recognition and on-the-job satisfaction.
Another tool, known at the Gratitude Tree, was invented by a former Hewlett-Packard engineer,2 and features a colorful, attention-grabbing display that reminds departmental and interdepartmental employees to recognize and appreciate one another. Alongside the tree, staff members and even visitors can express their gratitude to employees, teams and departments for a job well done. Expressing gratitude in this way lifts spirits and reminds all team members of what is important.
Author’s note: In my leadership role, I worked with a local sign company and produced a colorful design that matched our unit’s personality and displayed it in a public area that could be easily seen by employees coming to and from work, and also for patients and their family members. We placed blank vinyl stickers and a pen in a tray near the tree for anyone (patients, staff, leadership, and employees from other departments) to write a personalized message and place it anywhere they wish on the tree.
Aim for public recognition
The work provided by healthcare workers, including SP professionals, is challenging, so it is important for leaders to incorporate fun activities that help their teams bond with one another. Consider providing activities that allow employees to relax and cut loose briefly. This could include a pizza party or ice cream social, team-based games that test SP-related knowledge, and the development of creative inspiration boards that employees decorate for others to read and explore.
Daily or shift huddles provide additional opportunities to recognize employees and give recognition for the contributions and successes. It is helpful for employees to hear from their managers that they successfully meet expectations when performing their various roles. Providing this type of reassurance can reduce employee stress, reinforce performance expectations, and promote enduring engagement. Reduced stress and improved satisfaction can also lead to better work outcomes because calmer employees may make fewer mistakes.
During one of my department’s huddles, an employee whose performance stood out during the previous shift received a rubber wristband with the message, “Bring Your ‘A’ Game.” At the next day’s huddle, that employee had the opportunity to recognize another team member’s exemplary performance by passing the wristband to that colleague. This public display of acknowledgment allows employees to share witness of their peer’s excellent performance and build camaraderie among the team.
Another effective way to show employee gratitude is through an article or advertisement in a monthly newsletter. The newsletter, which can either be issued only to SP staff or included as part of a facility-wide correspondence, allows for leaders to communicate important information to more individuals, including employee of the month mentions, words of appreciation for new certifications attained by employees, promotions, and other noteworthy accomplishments. Employee spotlights helps everyone get to know each other on a more personal level.
If budgets allow, leaders may also consider providing monetary incentives (movie or car wash tickets, gift cards, etc.) or snacks and meals during targeted meetings, education and training.
When employees have a safe space to share ideas you communicate that you appreciate the time and effort they put into their work and the ideas they have. If you as a leader respond to their communication in a positive way or implement an idea they had, it is likely they will feel an even greater sense of loyalty in and pride for their organization. In addition, these opportunities show your employees that you are vested and care about them and their careers.
When employees feel appreciated and supported by their leaders and teammates, they are more likely to recognize and communicate their appreciation with their colleagues and customers. This, in turn, can have a significant impact on workplace culture, employee satisfaction and retention, and improved work processes and outcomes.
David Taylor III, MSN, RN, CNOR, is an executive healthcare consultant for Resolute Advisory Group LLC, based in San Antonio, Texas. He has served as a contributing author for HSPA since 2019.
References:
1. https://www.apa.org/news/press/releases/2012/03/well-being
2. https://www.bizjournals.com/washington/news/2018/07/18/a-local-woman-invented-a-gratitude-tree-now-shes.html
Who’s Responsible? Broken Processes Create More SPD Workload, Obstacles
By David Taylor III, MSN, RN, CNOR
This article originally appeared in the September 2022 issue of Healthcare Hygiene magazine.
Sterile processing (SP) operations and work systems are critical to hospital and healthcare organizations’ surgical and procedural programs, and SP professionals’ daily contributions factor heavily into patient outcomes. Despite this critical role in the surgical “ecosystem,” many SP departments lack the financial support, respect and understanding needed to help their technicians keep pace with customer and procedural demands and the rapidly changing aspects of surgical care.
The tasks for which SP technicians are primarily focused are numerous and include (but are not limited to) decontamination, inspection, assembly, preparation, equipment loading, process verification, sterilization or high-level disinfection, biological testing, distribution, and storage. Much of that work is a shared responsibility, even though the SPD is often expected to own those responsibilities solely. Too often, healthcare workers in procedural areas fail to understand and take ownership of their roles in the process, which can place additional burdens on SP technicians and impede their ability to process and manage instrumentation and medical equipment efficiently, safely and in accordance with the instructions for use and latest standards, guidelines and best practices.
Those in the SPD must interact with numerous other departments and professionals every day. SP professionals must have the support to ensure they have the proper tools, technologies, work environment, processes and procedures to manage their challenging workloads effectively each day and across all shifts. Reaching that goal requires an interdisciplinary commitment and an understanding that many departments are responsible for quality outcomes, not just those managing instrumentation in the SPD.
Water quality
Water quality is an organizational responsibility. If water quality standards fail to meet the end users’ needs, SP processes will break down, causing delays in instrument reprocessing. Often, those working in procedural areas do not focus on water quality and related issues—they just want to receive the instruments and equipment needed to perform their work. Still, it is necessary for all departments to know how water quality issues impact the workload within the SPD, and the instruments used to perform patient care.
If water is improperly treated, the impact on surgical instrumentation can be profound. If instrumentation is stained or showing signs of corrosion, for example, those devices must be pulled from circulation and promptly addressed to minimize patient risk. If there is no back-up set or instrument to replace the stained, corroded or otherwise damaged device, patient cases may be delayed or canceled altogether.
Point-of-use treatment
Point-of-use treatment, formerly referred to as point-of-use cleaning, refers to the removal of gross contamination (blood, tissue, bone, etc.) from reusable medical or surgical instrumentation in areas where patient care procedures are performed. This process should occur either periodically (during the procedure) or immediately after use, but before instruments are sent to the decontamination area in the SPD.
Removing gross soil—and moistening instrumentation with an approved wetting agent or even covering instruments with a water-moistened towel—helps prevent organic material and debris from drying on instruments, including lumened devices. It is much more difficult to remove organic material and debris from surgical instruments when they are allowed to dry, and residual soil can affect the efficacy of disinfection and sterilization. Removing organic debris also helps prevent the formation of biofilm, an accumulated biomass of bacteria and extracellular material that adheres tightly to a surface and cannot be removed easily. Note: The term “point-of-use treatment” was adopted in standards and guidelines in recent years because it better encompasses the activities that should take place at the procedure site to prepare instrumentation for transport to the decontamination area. “Point-of-use cleaning” terminology led to confusion because some assumed that thorough device cleaning should be performed in the surgical suite or other patient care areas following the procedure, which was not the intended purpose.
Once the instrumentation has been prepared for transport, it is critical that the devices be moved to the decontamination area immediately or as soon as possible to ensure the instruments don’t dry and can be subjected to prompt and thorough cleaning. Making the sterilization process less complex and more visible, managing interruptions during case cart preparation, improving communication with the procedural areas such as the OR, and improving workspace and technology design could further enhance reprocessing performance.
Low-quality, inadequate instrument repair
Organizations that adopt proactive instrument maintenance practices can positively impact physician and employee satisfaction, operational and capital budgets, instrument lifespan, and patient safety. Failure to support a robust surgical instrument repair and maintenance program can affect not only instrument/device quality but also the duration in which an instrument or set is unavailable due to extensive repairs or need for replacement.
The time it takes SP professionals to locate an adequate instrument replacement can also jeopardize patient safety. When instruments in use aren’t properly maintained, patients can experience longer procedures, extended anesthesia exposure and other negative outcomes. One example: if insulation in laparoscopic insulation becomes compromised, patients can experience life-altering thermal injuries.
Inadequate instrument inventory
Many organizations fail to invest in the appropriate number of instruments or sets to support their surgical programs and case volume. As a result, SP professionals must repeatedly reprocess the available sets in inventory, which again can result in case delays (or unsafe rushing of processes to meet demand), device overuse, added work, diminished staff and physician satisfaction, and increased patient risk. Additionally, healthcare organizations often fail to effectively address SP needs and growth patterns, surgical volume changes or the addition of new physicians and specialties.
To better support these programs, SP leaders must be kept informed of changes so they can prepare for and meet customers’ evolving needs. New physician recruitment is critical for advancing surgical programs and offering specialties communities need; however, those in the SPD cannot support physicians effectively and efficiently in the absence of proper information, tools, equipment, space/workflow, and staffing. SP leaders must also be involved in instrument purchasing discussions to ensure that the SPD has the necessary resources to process and manage the new devices or equipment according to IFU and standards.
As surgical procedures become increasingly complex, and the instruments required to support those programs become more sophisticated and challenging to clean, inspect and otherwise manage, it is imperative that organizations give SP professionals the deeper support needed to perform their daily responsibilities safely, effectively and efficiently.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as an HSPA contributing author since 2019.
Core Skills to Scale the Sterile Processing Leadership Ladder
By Tony Thurmond, CRCST, CIS, CHL
This article originally appeared in the August 2022 issue of Healthcare Hygiene magazine.
Sterile processing (SP) technicians observe their departmental leaders every day and witness their responses to challenges and how they support the team. They may also routinely see their fellow technicians rise up and assume an informal leadership role, sharing their knowledge and skill sets with their colleagues for the sake of quality and professionalism. This can then help light a spark for other SP professionals who may seek more responsibilities and, perhaps, even a formal or informal leadership role of their own.
SP professionals of all titles and tenures know the qualities they hope to see in a leader: strength in character and integrity, knowledgeable and eager to continue learning, willingness to mentor and encourage employee growth, empathetic to their team’s needs, and a willingness to roll up their sleeves and assist when needed. Those interested in attaining a leadership role will need to bring those same qualities to their own daily routines and hold themselves to the same high standards and behaviors. They should begin by examining the motivation behind their interest in a leadership position. If increased pay is the primary driver—as opposed to having a sincere desire to help support and serve the department in a way that promotes quality, safety and understanding, employers and teammates will see the truth. SP professionals should look inside themselves and ask questions that will help determine whether they are ready for this next move.
What follows are some leadership strengths and approaches that will help prepare SP professionals to climb higher in their careers.
Set a realistic pace. Climbing the SP leadership ladder is not a one-size-fits-all endeavor. The goal for some will be reach the very top and then keep aiming for even loftier career pursuits; for others, fewer rungs will need climbing to reach their goal. Regardless, the following traits and skill sets will help them succeed and build necessary trust.
Sharpen knowledge through experience. Sterile processing is a career where one does not just arrive and become an immediate expert. Knowing the role of the technician is the first step toward becoming a strong, effective leader. ANSI/AAMI ST79, Comprehensive guide to sterilization and sterility assurance in health care facilities, states that supervisory personnel should be competent and qualified for the supervision of all preparation and sterilization functions (i.e., decontamination, inspection, preparation, packaging, sterilization, storage, and distribution). Education, training and experience prepares them for this responsibility. ANSI/AAMI ST79 also recommends that the minimum recommended qualifications include certification, demonstration of job knowledge and adequate relevant experience in healthcare-related work. Knowledge of and experience with state and federal regulations — such as those from the Occupational Safety and Health Administration (OSHA) —are also required. Leaders, like all other SP professionals, must continue making education, knowledge and professionalism a top priority throughout their entire career.
Listen well. The ability to listen well is paramount to becoming a successful, respected leader. Hearing and listening are two very different abilities. A leader should be able to hear and listen as staff members come to them with a situation, challenge or idea. Leaders and would-be leaders must be prepared to ask the right questions and dissect the true meaning of what’s being conveyed. At times, employees may have an idea or solution they’d like to share—perhaps one that deviates from current practice or the status quo. Effective leaders are always willing to listen to new ideas and perspectives and be open to challenging “the way things have always been done.” Determining what the team needs and asks for comes from experience.
Hone decision-making skills: When situations within the department arise, solid decision-making skills are needed set the best plan into action. Many decisions that must be made will already be addressed in the department’s policies and procedures; however, some individuals may be asked to circumvent those policies and procedures to satisfy another person’s or department’s needs. It will always be necessary to stick to safe, standards-based processes and best practices and explain why some requests or proposed changes cannot be made. Simply stating “because it is in our policy” will not be effective or adequate. Explaining why the process must be followed (and backing that with standards, best practices, instructions for use, and internal policies and procedures) will help sidestep any risks and reaffirm one’s commitment to doing what is best for the patient.
Serve as a trusted advocate. Patient safety must be every healthcare professional’s priority and must always serve as the foundation for all that is done within the department. Maintaining that focus will guide SP professionals to become the best leaders possible. Effective leaders also advocate for their employees. Telling the operating room team that the SP team accomplished a monumental task and was able to prepare everything needed in a short period of time, for example, helps build the respect and interdisciplinary teamwork, and shows the employees that they are supported and appreciated. The same is true when problems arise. If an employee is berated by a customer for a mistake or an unwillingness to rush a process to meet unrealistic turnaround demands, for example, intervening on the employee’s behalf (or guiding them toward an effective discussion/response) can help improve future service delivery and expectations.
Demonstrate fairness: Leaders must always strive to treat each member of the team as equally valuable contributors. Frequent rounding of the department is a great way to engage and interact with all members of the team and to keep a steady finger on the pulse of what’s happening each day and with all employees.
Maintain integrity: Integrity is an essential trait for every SP professional, regardless of title. A leader whose integrity never wavers helps clearly show what is expected of the entire team, regardless of the challenges that may arise. It also demonstrates that separate rules don’t apply for leaders and technicians—a point that helps instill trust and mutual respect. A leader that possesses and demonstrates strong integrity, respect and a desire to treat everyone fairly and equally creates a healthy atmosphere of “psychological safety.”
As one aims to climb the SP leadership ladder, practicing and perfecting these traits and skills will not only positively define them as a professional, but as an effective leader. Good leaders will always be needed and if an SP professionals is willing to put in the work, their time will certainly come.
Tony Thurmond, CRCST, CIS, CHL, is central service manager at Dayton Children’s Hospital.
SPD Transparency Delivers Facility-Wide Benefits
By Tony Thurmond, CRCST, CIS, CHL, FCS
This article originally appeared in the July 2022 issue of Healthcare Hygiene magazine.
Every sterile processing (SP) professional understands that cleaning instruments properly requires meticulous adherence to instructions for use and industry standards and diligent inspection to ensure any hidden bioburden is detected and addressed. Doing so also helps ensure that devices function well, consistently and as intended.
If SP technicians could hold up each instrument and see through it as if it were glass, that transparency would allow bioburden to be easily seen, which would facilitate proper cleaning. Fewer errors would be made, technicians’ jobs would be significantly less challenging, and patient safety and infection prevention would be improved. Certainly, clear instruments aren’t available; however, if SP professionals were to strive for greater transparency in their roles and within the workplace, positive developments would surely result. Being able to clearly see what is expected of SP technicians and their teammates is a crucial factor in the department’s success, as is communicating more transparently with SPD customers and instrument end users.
Workplace transparency can be loosely defined as functioning in a way that creates openness and honesty between managers, employees and other departments. Promoting and practicing transparency in the workplace pays big dividends but it takes planning and commitment. Transparency enhances trust, improves communication and employee participation and helps instill a desire to go the extra mile. This it is not a simple switch that gets flipped on but a day-by-day process that is nurtured by all sides in a quest for improved, bi-directional communication, planning and outcomes. How a manager defines and pursues transparency in the workplace may differ, but the underlying goal remains the same: to unite teams to openly discuss concerns, issues or ideas.
Determine the details to share. Managers know which types of information can and should be shared with all employees. Examples include short- and long-term goals and expectations for the department; daily needs to meet schedules/customer demands; quality improvement initiatives; policy and procedure updates; standards changes; new equipment and training needs; and so on. Conversely, some information may only need to be shared with mid-level management to help prepare them for an upcoming change or a plan of action that has not been finalized. Such instances do not erode departmental transparency; it’s simply unnecessary or unhelpful to bog all employees down with details and information that doesn’t directly affect them. Each manager should determine the level of transparency/communication for each circumstance and then filter information to employees when more details can or should be shared. If questions arise and managers lack complete details or are otherwise unable to share more at that time, it is best to be honest and explain that more details will be given later. Similarly, when an SP leader or technician fields a question from a customer that cannot be immediately answered, it is best to admit that more research is needed before an appropriate answer can be given.
Promote team integrity. Integrity it can be loosely defined as doing the right thing at all times, even when no one else witnesses it. Leaders and employees from other departments are expected to demonstrate integrity and they expect the same of those in the SPD. Admitting a shortcoming or taking responsibility for an error is more admirable than denying personal involvement and it’s an action that will be far more respected by the SPD’s colleagues and customers. When SP technicians and leaders consistently act with integrity and professionalism, fewer issues occur, problems and risks are mitigated, and teams can work collaboratively toward an effective resolution. It is also important that technicians not only demonstrate integrity with their own actions but encourage and promote it in their teammates. If they see someone taking a shortcut, for example, they should be politely reminded of the proper way.
Educate about the SPD. Those in the SPD should share their story and educate others about their many roles, responsibilities, successes and challenges—and how the way instruments are handled during and after use can affect reprocessing outcomes. If user departments fail to perform point-of-use treatment on instruments or transport devices improperly, for example, or they routinely schedule back-to-back procedures even though the facility lacks adequate instrumentation inventories, staffing and processing equipment to keep up with the demands safely, negative outcomes can result.
Cultivating a culture of transparency is vital in the healthcare environment where patient lives are stake, and it’s certainly essential in the SPD. Trust and respect are gained when information is shared openly and honestly, and when all employees take ownership for their mistakes and shortcomings. Further, positive outcomes and improved processes result when interdepartmental teammates understand others’ roles and challenges and work together on improvements.
Tony Thurmond, CRCST, CIS, CHL, FCS, serves as central service manager for Dayton Children’s Hospital. He is a columnist for the Healthcare Sterile Processing Association (HSPA) and past-president, and currently serves as a director on the HSPA board. He received his HSPA fellowship in 2021.
Sterile Processing Department PPE: A Shared Responsibility for Infection Prevention and Safety
By David Taylor III, MSN, RN, CNOR
This column originally appeared in the June 2022 issue of Healthcare Hygiene magazine.
The Occupational Safety and Health Administration (OSHA) requires employers to evaluate workplace hazards and gauge the risk of employee exposure. Employers must also implement a plan to mitigate exposure to hazards, select the appropriate protective equipment and ensure workers use those items to avoid exposure.1
Personal protective equipment (PPE) minimizes exposure to hazards that can cause serious workplace injuries, illnesses and death. These injuries and illnesses may result from contact with chemical, radiological, physical, electrical, mechanical or other workplace hazards or the spread of infection or illness. Examples of sterile processing-related PPE include but are not necessarily limited to the following:
• Scrub attire (depending on facility policy, a cover gown/lab coat may be worn to protect scrub attire when leaving the department for another area in the same facility)
• Moisture-resistant gowns and/or aprons
• Masks, face shields and/or goggles
• Gloves (of appropriate type, length and size for the task and individual employee)
• Shoe covers (to be worn with sturdy, non-skid shoes; to prevent contamination, it is wise to have shoes dedicated to the work area and not worn outside)
• Disposable, bouffant-type head covering to cover all head hair, minus eyelashes and eyebrows. Note: Skull-types caps are not recommended because they may not cover all head hair. Facial hair should be fully covered with an appropriate beard cover.
Healthcare organizations are responsible for providing the appropriate types of PPE as well as adequate supply levels and sizes for employee use. Since the COVID-19 pandemic, ensuring adequate supplies has been challenging due to supply chain shortages and increased supply demands; however, healthcare organizations must work diligently and proactively to help ensure they are doing everything possible to provide employees with the PPE needed to stay safe in the workplace. On April 1, 2021, a California law took effect requiring that hospitals maintain a three-month supply of PPE and create and maintain a three-month stockpile of N95 respirators, gowns and other PPE.2
While it is vital that healthcare organizations are held accountable for protecting their employees, employees also share in the responsibility of proper, consistent PPE use.
Donning and doffing steps
Careful donning and doffing of PPE in the correct sequence is essential for preventing contamination and exposure. Note: For a downloadable/printable reference on proper PPE use, including donning and doffing diagrams, visit: www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf.
Unfortunately, some employees fail to perform proper hand hygiene after wearing PPE, neglect to wear proper eye protection or fully cover their hair or fail to use gloves properly when handling items containing blood or body fluids. Observations of healthcare workers in a university hospital in Germany revealed deficiencies in the use of recommended PPE among all observed healthcare workers while caring for COVID-19 and non-COVID-19 patients.3 Deficits in everyday handling of PPE have been observed previously, especially in regard to fitting and correct sequencing and use, and were found in 90 percent of personnel.3.4 Most commonly, errors occurred in the correct removal of gowns (65 percent) and contact with potentially contaminated surfaces (48 percent).4
In my consultant role, I have seen some alarming PPE-related missteps in various healthcare facilities. The following photos were taking over two days at a prominent teaching hospital in the Northeast. PPE was in disarray and employees spent several minutes sorting through the mess to locate the appropriate PPE when entering their decontamination area. When needed PPE was unavailable, they would search other areas of the hospital to try and locate the correct item. Employees at this facility were forced to use PPE that was either nonexistent, old and in disrepair, or improvised with other items that were often inappropriate for the task at hand. One example: when removing the rack from their steam autoclaves, employees used an old silicone protective glove designed for home use (or a hand towel) to protect themselves. All these silicone gloves in use in the department were torn and, when asked, employees admitted several co-workers had suffered burns while at work—and management was aware.
Assessments are essential
All healthcare organization and departmental managers must ensure that PPE is provided and used in accordance with the latest industry standards, guidelines, best practices, instructions for use, and policies and procedures. Diligent assessments of PPE supplies, storage and organization, donning, doffing and disposal should be conducted to keep employee and patient safety a top priority (this should also entail ensuring that proper sizes are available to meet all employees’ needs).
If supply and usage gaps are identified, additional employee training should be provided, ongoing assessments should be conducted, and managers should work closely with their materials management and infection prevention partners to help ensure all employees in the department have ready access to all necessary PPE.
David Taylor III, MSN, RN, CNOR, is an executive healthcare consultant for Resolute Advisory Group LLC, based in San Antonio, Texas. He has served as a contributing author for the Healthcare Sterile Processing Association (HSPA) since 2019.
References:
1. COVID-19 - Control and Prevention - Healthcare Workers and Employers | Occupational Safety and Health Administration (osha.gov)
2. New law requiring hospitals to maintain a three-month supply of PPE takes effect April 1 | National Nurses United
3. Adherence to personal protective equipment use among healthcare workers caring for confirmed COVID-19 and alleged non-COVID-19 patients | Antimicrobial Resistance & Infection Control | Full Text (biomedcentral.com)
4. Personal protective equipment doffing practices of healthcare workers: Journal of Occupational and Environmental Hygiene: Vol 16, No 8 (tandfonline.com)
A Case for Cost Analysis & SBARs: Ensuring SPD Gets What It Needs
By Julie E. Williamson
This column originally appeared in the May 2022 issue of Healthcare Hygiene magazine.
Many sterile processing departments (SPDs) struggle to attain the appropriate resources, products, staffing and equipment to ensure their teams are able to manage their processes and practices safely and efficiently. But experts assure that cost analyses, when implemented executed properly, can help sway the C-suite’s purchasing decisions in a positive direction.
As Jamie Zarembinski, clinical educator for sterile processing for Key Surgical, pointed out during her April 25 session at the 2022 HSPA Annual Conference, an effective cost analysis can significantly help SP leaders leverage data to help paint a clear picture of what the SPD needs and why allocating the resources can help the department, its customers and patients and the collective healthcare organization.
“The pressure is on the SPD to not only say ‘We need this,’ but also to share why,” she said, noting that the right information presented on a cost analysis sheet can help set SP leaders up for success when meeting with those in the C-suite, especially when the request can result in improved safety, infection prevention and process efficiency.
Cost analysis is more than just asking for money for resources, however. Zarembinski reminded that it is a tool that helps organizations analyze and make informed decisions related to potential new products and requested resources. Put simply, it’s a process that helps decisionmakers quickly determine the value of a particular request. “It isn’t enough to just say, ‘I want that.’ A cost analysis can help you build a story, so you know where your money is going,” she reasoned. If you can’t answer the question, ‘why do we need it?’ you won’t be able to get to the how.” To start, SP leaders can work on resource requests with their teams to determine wish lists and perceived necessities for the department. Zarembinski advocates for the creation of vision boards, allowing all team members to express visually and in writing their wants and needs, regardless of budget. “Imagine $10 million was put into your budget and then determine what you want most and why. There is no goal too big for you to put on a vision board.”
From there, the team should explore their existing processes and practices to ensure safety and efficiency are being prioritized. Without that, improvements won’t be made, regardless of the new product, piece of equipment or resource acquired. “You may want five new employees or another washer, for example, but you really need to assess your practices and [rely on standards, best practices and instructions for use] to help determine whether something else is going on that won’t be fixed by some new product or equipment. Otherwise, the new resource will just be a Band-Aid, and nothing will get better.”
Hit the High Notes
While telling the true story on a cost analysis sheet is essential for stacking the acquisition odds in the SPD’s favor, experts stress the importance of sticking to vital details, eliminating extraneous information that could muddy the request, and using data and scores that can be readily attained on the internet (such as the facility’s healthcare-associated infection scores provided by the Centers for Medicare & Medicaid Services) as a driving force behind the need.
A cost analysis sheet is relatively basic, and it should clearly help the C-suite determine the return on investment and value proposition for the facility. If a new washer is being requested, for example, it would be helpful to detail the department’s throughput and procedural volume to help establish the need and impact of the purchase—as well as the risks to the organization and patients if the acquisition does not happen (e.g., inability to meet surgical procedural volume, perils of operating a dated or poorly performing unit, and the increased risk of healthcare-associated infections as a result).
One common and effective resource request technique is known as the SBAR, which stands for Situation, Background, Assessment, and Recommendations. The Situation portion is a brief and factual statement of the problem (i.e., a dated and poorly functioning washer that cannot keep up with the facility’s demands and patient safety needs). The Background is used to highlight evidence-based guidelines and best practices (essentially explaining that without the adequate tools, equipment and/or well-trained technicians, the SPD cannot safely and effectively perform its vital roles). The Assessment portion allows SP leaders to share and interpret their findings. This should be written so that a person who doesn’t know much about day-to-day SPD operations can easily understand the basis of the concerns, noted Marie Brewer, CST, CRCST, CIS, CHL, CER, GTS, CLSSGB, Sterile Processing Manager for St. Luke’s Hospital.1
The Recommendations section is the “show me the money” portion—the full-time equivalents, tools, machines and products SP professionals need to follow IFU and protect patients and staff members.1 Proper documentation is also essential for making the case to the C-suite, Brewer explained, adding that photographs can be an effective way to document a problem and help connect the decisionmaker with the pain points of the Background section. She further stressed how citing or attaching evidence-based guidelines and publications that support the need for what is being requested can have a positive impact on the decision to sign off on a request.1
Turning a No into a Yes Sometimes, the C-suite declines resource requests, regardless of how well the request was executed. Fortunately, perseverance can pay big dividends for SP leaders.
Zarembinski shared a story of one manager who, after being promptly turned down for a request for a new cart washer, immediately concluded the resource request meeting by inviting the CFO to the SPD to see the department in action. The CFO accepted the invitation and after spending just a brief period in the SPD and witnessing the processes—and bottlenecks associated with wiping down the case carts—responded quickly to ensure funds were allocated for a new cart washer.
“Sticking your neck out a little and asking others to come in the department so they can see and understand the processes a bit and what goes on there can be very effective. Even if you get a thousand no’s, you can still get that yes.”
Julie E. Williamson serves as editor and communications director for the Healthcare Sterile Processing Association (HSPA).
SPD Renovation: Building in Quality, Safety, Infection Prevention
By David Taylor III, MSN, RN, CNOR
This column originally appeared in the April 2022 issue of Healthcare Hygiene magazine.
Despite the critical role sterile processing departments (SPDs) play in the delivery of safe, high-quality service and patient care, many healthcare organizations are making the mistake of overlooking these departments for renovations or new construction. The space an SPD has available can have a significant impact on its operations. Far too many healthcare organizations have grown in scope and size over the years while the SPD’s space needs have been neglected. If health systems fail to support SPDs’ role and build adequate space to allow SP professionals to properly handle their daily workloads, it’s not just SP professionals who will be adversely affected. Healthcare customers such as the operating room and Endoscopy can experience service challenges that lead to procedure delays and poorly functioning or contaminated instruments, and patients can experience extended procedure times, potentially life-threatening infections and other negative outcomes.
When it comes to SPD renovation or expansion, facility administrators face some difficult questions, including whether they should allocate financing to improve the SPD space or dedicate funding for other potential revenue-generating specialties (e.g., emergency department, endoscopy, interventional radiology). Whatever the decision, organizations must realize that failing to include SPD in their expansion or renovation plans is a short-sighted decision that will increase the odds for negative outcomes. Expanding the department’s capacity (square footage) and improving the infrastructure to support modifications in the SPD will improve efficiencies within the department (and downstream) and help improve processes in the OR and other procedural areas, thereby creating greater revenue streams.
Some of the challenges administrators must consider and address include:
• Advances in surgical approaches and new or specialized instrumentation and technology, all of which require specific sterilization equipment and processes (e.g., robotic-assisted surgical systems)
• Changes in recommended practice guidelines and standards by industry organizations and regulatory agencies
• Hospital or health system growth
• Increased case volumes or the addition of new surgeons and specialties, resulting in increased tray volume
• Increase in surgical schedules (extended hours and weekend schedules)
• Inadequate and deteriorating infrastructure (mechanical, electrical, plumbing, technology) that prevent the installation of newer, high-capacity equipment and technologies
• Inadequate space and inefficient workflow processes
SPD zone planning
How an SPD is laid out can have a significant impact on its productivity. Most SPDs are configured in either a two- or three-zone layout that follows a dirty-to-clean path and complies with industry standards and guidelines or regulatory practices. Departmental configurations vary and can include straight or L-, U- or Z-shaped flow patterns. Unfortunately, too many SPDs have been squeezed into spaces that may have been appropriate many years prior but are now inadequately sized to accommodate their organization’s growing needs.
SPD layout must provide physical separation between work areas to allow technicians to handle soiled material effectively and safely, clean, prepare, assemble, sterilize, store, and dispatch goods. SPD space planning should be based on the roles and responsibilities the department is expected to assume. Some crucial questions to be asked include:
• How many surgical (and other) procedures are projected daily and how many instrument sets are projected to be reprocessed by SP daily?
• How many procedures require vendor tray support?
• How many case carts, transport carts and/or container racks will be reprocessed daily?
• Will the SPD reprocess rigid and flexible endoscopes from non-surgical areas?
• Will pass-through sterilizers and cart washers be used?
• Will the SPD be responsible for durable medical equipment and supplies?
• Will the SPD manage restocking and reassembly of implants?
• How many full-time equivalents (FTEs) will work on the SPD’s peak shift?
• Is an area dedicated to donning and doffing personal protective equipment (PPE)?
• Are there dedicated receiving, breakout and inspection areas?
• Is an area dedicated to chemical storage?
• Is there adequate office space (private, shared, cubicle) and conference and classroom space?
• Is there dedicated space for instruments, containers and equipment in need of repair?
• Is a transitional space available for receiving and delivery?
• Is a dedicated holding and pick-up space available?
• Is enough storage space allocated for sterile consumables and durables?
• How is the department managing case cart assembly and dispatch?
Soiled drop-off/transition
Transition zones are often inadequate in the SPD, with many sharing space with the decontamination room, which creates areas of exposure and risk. Separating the main corridor from the decontamination workspace accommodates the temporary holding area for soiled carts, totes, medical equipment and vendor trays—without directly entering the decontamination workspace. More importantly, it creates a buffer zone that is a perfect location for instrument and equipment inventory and electronic tracking systems (delivery and pick up). Note: It only takes a few vendors to claim instruments and implants are missing from their trays to understand that the investment in such a dedicated space will pay big dividends.
Decontamination area
The decontamination area is used for unloading case carts, containers, etc. and cleaning, preparing and inspecting instruments. This area’s size should be based on the expected production requirements and range from small (approximately 600 square feet) to large (over 900 square feet). For example, SPDs that process fewer than 150 instrument trays daily may require a smaller footprint, while those that process 150 to 400 trays or more will need additional space. Keep in mind, surgical programs are expected to grow exponentially as the U.S. population ages, and there will be a growing need for more equipment to meet the demands and keep up with technological advancements. If an SPD is responsible for endoscope processing, additional square footage will likely be needed. Equipment needs (including automated cart washers) will help dictate the proper size of a space; it is important to consider the manufacturer, type and quantity of the equipment (currently in use and what may be needed in the future).
The decontamination area should include:
• A handwashing sink with emergency eyewash fixture
• Counter space or storage racks to prepare instruments and equipment for their first phases of cleaning
• At least one pass-through window large enough to accommodate all instrumentation and equipment
• A pass-through door for durable medical equipment
• Dedicated space if endoscope reprocessing will occur
Assembly area
SPD leaders should understand the various aspects the assembly area will accommodate. Like in decontamination areas, space needs will depend on the number of sets processed in a 24-hour period. Consider space needed for an instrument washer and pass-through window, an instrument unloading and preparation station, an instrument set assembly area, a workstation for inspection and quality assurance validation, a workstation for containerizing and wrapping sets, a dedicated space for telecommunication, computers, barcode readers/trackers and printers, and storage for supplies, instrument containers, wrappers, pouches, clean linen, etc.
Sterilization area
When considering space needs in this area it is important to know the type of equipment that will be processed, and which sterilization modalities will be used. Additionally, space should be allocated for biological monitoring and proper documentation. Again, space needs in this area will depend on the number of sets processed over 24 hours.
All-inclusive approach
Healthcare organizations must understand the SPD’s needs and workflow demands before determining renovation needs and next steps. When considering a renovation project, relocating the department, or building a new facility, healthcare leaders should first consider creating new-and-improved processes and workflows to help immediately resolve inefficiencies (this should always be done before beginning a construction project). When a renovation, expansion or new build is in order, a collaborative approach is vital and should include strong SP representation and other stakeholders (e.g., nurses, doctors, leadership, facilities and maintenance department, and construction personnel).
Collectively, the team must determine whether the project can be completed without disrupting services. It will also be necessary to update preference cards; determine instrumentation inventory for each surgeon and specialty (based on current and projected volumes per specialty); reassign, rebuild or decommission instrument sets from surgeons no longer working at the facility; evaluate instrument sets with each surgeon and determine changes in their practice that could impact instrumentation and equipment needs; standardize trays (adding new instrumentation as needed, removing excess instrumentation to speed production, and meeting set weight limits); evaluate containerization versus wrapping of instrument sets; review vendor tray opportunities (ensuring appropriate space for instrument arrival/check in and pickup/check out and removing excessive vendor tray storage.
When determining equipment needs and proper layout, it is also vital to identify instrument workflow and equipment paths, standardize workstation location and layout, and determine supply and equipment needs for inspection and assembly. Determining the SPD’s mission and fully understanding the customers they will serve—all while anticipating future space and resource needs—is also essential, as is a thorough evaluation of storage needs and options (e.g., vertical versus horizontal and automated versus manual). Facilities will also need to consider how the SPD will transport clean and dirty instruments (e.g., use of dedicated elevators or ensuring adequate staffing to facilitate transport to and from user areas), how many FTEs are needed to perform effectively in the new space, and whether the new SPD will require automated processes and perhaps even robotic assistance.
In conclusion, many factors come into play when considering SPD renovations or new construction. A team approach with representation from SP professionals and other stakeholders is critical to ensure that the SPD’s current and future needs are understood and supported. When organizations take time to carefully examine current processes, equipment and spaces and future requirements, they will experience a far greater return on investment.
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as an HSPA contributing author since 2019.
Current, Accessible Safety Data Sheets Critical for Safe SPDs
By Tony Thurmond, CRCST, CIS, CHL, FCS
This column originally appeared in the March 2022 issue of Healthcare Hygiene magazine.
Quality- and safety-focused sterile processing (SP) professionals arrive at work each day with the intent of performing their many critical duties to the best of their ability and with the hope that no safety issues befall them or their teammates. They don’t go to work, change into their personal protective equipment (PPE) and say to themselves, for example, “Today is the day I will receive a dangerous chemical burn.”
But what if today actually is the day a chemical-related safety event occurs? Is there a proper plan in place for managing the situation and preventing future injuries and incidents? Make no mistake, the numerous chemicals, sterilants and products SPDs rely on daily can present a serious safety threat, and everyone working in the department must know how to protect and care for themselves or their colleagues if an accident or exposure occurs. Knowing where to look for guidance is paramount for stemming the risks—and ensuring ready access to manufacturer safety data sheets (SDSs) should rank among the SPD’s highest priorities.
The Occupational Safety and Health Administration (OSHA) requires each employer to make SDSs available for every chemical used in a working facility and department. SP leaders must ensure all SDSs are readily accessible to their employees and that all team members know how to access SDSs for the products used in their department. If the SPD has a manual for the chemicals used, it must be readily accessible, and if SDSs are kept on a computer, all employees should have computers available for quick access and reference. All SDSs must be current and include the most up-to-date information (chemical manufacturers are required to provide an SDS for every chemical produced, and these vendors must update their SDSs as often as required to remain OSHA compliance). SP leaders should regularly review SDS content to ensure all employees are using the latest version.
SDS versus IFU
To promote a safe workplace, it’s essential that SP professionals understand the differences between SDSs and instructions for use (IFU) and not confuse their purpose. IFU provide directions of how to use the product whereas SDSs are directives that guide through proper storage, exposure, care and management of an exposure, and proper disposal of the chemical.
According to OSHA’s Hazard Communication Standard, SDSs must be presented in a user-friendly, 16-section format.
Section 1 – Identification: Identifies the chemical on the SDS as well as the recommended uses and supplier contact information.
Section 2 – Hazard(s) identification: Includes chemical hazards and warnings related to those hazards.
Section 3 – Ingredient composition/information: Identifies product ingredients outlined on the SDS, including impurities and stabilizing additives. This section also includes information on substances, mixtures and all chemicals where a trade secret is claimed.
Section 4 – First-aid measures: Describes the initial care that should be administered by untrained responders to an individual who was exposed to the chemical.
Section 5 – Fire-fighting measures. Includes recommendations for fighting a fire caused by the chemical, including suitable extinguishing techniques, equipment and fire-related chemical hazards.
Section 6 – Accidental release measures: Provides recommendations on appropriate response to spills, leaks or releases, including containment and clean-up practices to prevent or minimize exposure to people, property or the environment. It may also include recommendations that distinguish between responses for large and small spills (for incidents where spill volume has a significant impact on the hazard).
Section 7 – Handling and storage: Provides guidance on safe handling practices and conditions for safe storage of chemicals (including chemical incompatibilities).
Section 8 – Exposure controls/personal protection: Indicates exposure limits, engineering controls and PPE measures that can minimize employee exposure.
Section 9 – Physical and chemical properties: Identifies physical and chemical properties associated with the substance or mixture.
Section 10 – Stability and reactivity: Describes reactivity hazards of the chemical and the chemical stability information.
Section 11 – Toxicological information: Identifies toxicological and health effects information or indicates that such information is unavailable (includes routes of exposure, related symptoms, acute and chronic effects, and numerical measures of toxicity).
Section 12 – Ecological information: Provides information to evaluate environmental impact of the chemical if released into the environment.
Section 13 – Disposal considerations: Provides guidance on proper disposal practices, recycling or reclamation of the chemical(s) or its container, and safe handling practices.
Section 14 – Transport information: Includes guidance on classification information for shipping and transporting of hazardous chemical(s).
Section 15 – Regulatory information: Identifies safety, health and environmental regulations specific for the product that is not indicated anywhere else on the SDS.
Section 16 – Other information: Indicates when the SDS was prepared or when the last known revision was made and may also state where changes have been made to the previous version.
When evaluating and purchasing a new chemical product, the SDS should be carefully reviewed to ensure the department can meet the storage requirements and also ensure the department has the required PPE for use with the chemical. Chemical spill kits must also be readily available and visible to departmental staff. Because the IFU provides directions for proper product use, and the SDS provides directions for the exposure or spill of a chemical product, there will be times when SP professionals will need to refer to both. SPDs would benefit by holding in-services to discuss new products, explore details of the SDS and address employee questions and concerns.
SPDs must strive to keep employees safe. Engaging in safe practices and ensuring all SP professionals understand the steps involved in mitigating risks and addressing safety incidents when they occur is essential for positive outcomes—and the SDS is an essential tool to help meet that critical goal.
Author’s note: SDSs’ 16 sections provide an abundance of information that can seem daunting to the reader/user. These data sheets can be broken down further to simplify reading. For example, Sections 1–3 describe basic chemical details; Sections 4–8 describe recommended actions in the event of an accident; Sections 9–11 describe technical details such as nature of the material and where the hazards exist; and Sections 12–16 contain information for specific needs. I advocate reviewing the complete SDS, but it is vital to focus on sections 1–8 because these sections are needed for preparation or quick action.
Tony Thurmond, CRCST, CIS, CHL, serves as central service manager for Dayton Children’s Hospital. He is a past-president and current board member of the Healthcare Sterile Processing Association (HSPA). He attained his HSPA fellowship distinction in 2021.
Sterile Processing: Tying Customer Service to Care Quality, Safety Mission
By David Taylor III, MSN, RN, CNOR
This column originally appeared in the February 2022 issue of Healthcare Hygiene magazine.
For sterile processing departments (SPDs), customer service revolves around their skilled technicians providing instruments and equipment that are on time, complete, well-functioning, clean and sterile (or high-level disinfected).
SPDs are highly complex departments that manage many responsibilities to service numerous areas of the hospital. In fact, the SPD’s scope of responsibilities often extend far beyond the operating room (OR) to include all procedural areas, including but not limited to the Emergency Department, intensive care unit, Endoscopy, and on- and off-site surgery centers and clinics. Many SP professionals are also responsible for the cleaning, sterilization and maintenance of durable medical equipment, maintenance of crash carts and trauma and hemorrhage carts, restocking of supplies, and material management support for the organization. Each area served by the SPD has different needs and expectations from the SPD, which can make SP professionals’ jobs even more challenging.
For SP professionals to best meet their various customers’ needs and expectations, it is important that their customers understand the processes and time constraints associated with fulfilling daily requests. If an SPD customer schedules multiple procedures in a particular day but only has enough instruments or equipment to comfortably support half that scheduled amount, expedited turnover of those devices could likely be required. The question is can SP professionals realistically turn over those items in a safe way, while still supporting the customer’s needs and keeping schedules on track (and on time)? Doing so might be unrealistic, depending on the time of day, whether enough technicians are available to handle the workload, or whether the equipment needed to reprocess the devices is available and not already in use. Sometimes, customers may assume that their needs take priority over others’, and they may fail to understand all that goes into fulfilling their request; therefore, it is necessary for SP professionals to manager customer expectations through improved communication and as-needed education that is rooted in the latest standards, best practices and the facility’s policies and procedures.
Reprocessing instrument sets, rigid and flexible endoscopes and other reusable devices and equipment requires multiple steps that cannot be rushed or skipped. Each step must be performed in accordance with the manufacturer’s instructions for use (IFU), and enough time and resources must be allocated to safely and consistently allow technicians to perform all steps (e.g., sorting, cleaning, drying, inspection, preparation and packaging, sterilization, distribution or storage). Also to be considered: the need for manual precleaning; soaking time requirements; number of times channels must be brushed; drying times; and number of cycles specific items must go through an automated process, to name a few. It is essential that SP technicians and their customers recognize that even the initial inspection of the item—to ensure it is in good working order and not received damaged—takes time, and that the decontamination process can take numerous steps, each requiring specific time requirements or motions (brushing a channel 10 times, for example).
Cleaning instruments to remove all gross contamination (e.g., blood, bone, proteinaceous material) is a critical step and requires keen attention to detail. For some instrumentation, ultrasonic cleaning is a necessary next step to help remove contaminants that manual cleaning may have missed. Placing instruments in the washer-decontaminator is the final cleaning. Note: For endoscope reprocessing, once the manual cleaning and flushing steps are done, many hospitals activate their “Cleaning Claim” cycle in their automated cleaning equipment. The cleaning claim is an automated process that mimics the manual cleaning process. This added step adds time to the process but puts endoscopes through a more rigorous process to ensure proper cleaning and high-level disinfection are met.
All reprocessing steps take time and are dependent on the size, complexity and fragility of the instruments and equipment, as well as each SPD’s available resources (instrument inventory, reprocessing equipment availability, and staff availability). Inspection and assembly of an instrument set will also add time to the process, and these steps must also never be rushed or skipped. Further, sterilization and high-level disinfection cannot be rushed or interrupted; when that happens, the process must start over. Other steps that need to be factored into the reprocessing timelines and customer expectations include the need for proper documentation, cooling, and storage.
Additional factors that can add time to these processes are dependent on mechanical mechanisms. For example, the IFU provided with a certain brand of flexible gastrointestinal (GI) endoscope requires multiple specific steps (i.e., once the procedure is completed, the first step in the cleaning process is to wipe the outer portion of the endoscope and suction pre-cleaner through the channels. This starts a one-hour timer. If the endoscope is not submerged and cleaning begins, that endoscope will be required to soak for between one and 10 hours before the cleaning process can begin).
SP leaders should invite their customers to tour the SPD and spend quality time in each area to see firsthand all the steps required to process an instrument (from the moment an item arrives in decontamination to the time it is ready for pickup or delivery to the user area). Each SPD area must tackle specific sequential tasks before the next step can begin. Educating customers about each of these steps and their critical importance, as well as the type of equipment and resources needed to accomplish each step safely and effectively, is a valuable approach. It is also beneficial for SP leaders to introduce the customer to the technicians responsible for processing their items. Doing so helps put a face to a name and strengthens professional relationships and accountability.
The key to creating positive, enduring relationships with SPD customers is to build bridges through education and ongoing, two-way communication. SP professionals and their customers will gain a better understanding of and appreciation for one another’s needs and will help keep expectations in check and aligned with safety and quality goals. Having customers that better understand what goes into reprocessing will also help SPDs and their customers determine resource shortcomings, which can help prioritize future needs (such as additional equipment and instrumentation) to ensure items can be processed safer and more effectively and efficiently.
David Taylor III, MSN, RN, CNOR, has served as a contributing columnist for HSPA since 2019. He is the principal of Resolute Advisory Group LLC, a healthcare consulting firm in San Antonio, Texas. Questions or comments about this article can be directed to david@resoluteadvisorygroup.com or Julie Williamson, HSPA’s Editor and Director of Communications (jwilliamson@myhspa.org).
Overcoming the Challenges of Loaned Instrumentation
By Julie E. Williamson
This column originally appeared in the January 2022 issue of Healthcare Hygiene magazine.
Managing loaned instruments and trays safety, effectively and efficiently is a common struggle for many sterile processing (SP) professionals—and one that has grown more problematic due to complex and often heavy sets filled with complicated and sophisticated instrumentation.
Proper care, processing and handling of these devices from the time they are received at the facility to when they are picked up after their use hinges on proper planning and the provision of adequate resources. This includes having enough time, staff, processing equipment, supplies and storage space, as well as ready access to manufacturers’ instructions for use. Beyond that, facilities will need multidisciplinary involvement and adherence to policies and procedures specific to loaned devices (e.g., from those in the SPD, operating room, purchasing/materials management, and infection prevention), reminds Damien Berg, BA, BS, CRCST, AAMIF, vice president of strategic initiatives for the Healthcare Sterile Processing Association (formerly the International Association of Healthcare Central Service Materiel Management). In his previous sterile processing leadership roles, most recently as regional manager of sterile processing for University of Colorado Health, he saw his fair share of loaned instrument-related challenges and he and his interdisciplinary teammates worked hard to overcome them.
“Over the years, I saw how important it was to have policies and resources in place to help mitigate some of the problems associated with loaned instruments,” Berg said, noting that some of the hospitals his team supported averaged 40 to 50 surgical procedures a day, many of those orthopedic and spinal procedures that involved an overwhelming number of loaned instruments.
Although managing loaned instruments may seem relatively simple on the surface (with items delivered in advance of the procedure and then picked up by the vendor(s) afterward), the process is often quite complex. There are many opportunities for miscommunication and errors if prudent policies aren’t adopted and all stakeholders aren’t committed to ensuring successful receipt and post-procedure pickups of loaned devices.
Berg reminds that loaned instruments don’t always come from vendors or distributors; they can also come from sister facilities. This allows different hospitals or surgery centers within the same organization to share inventory and reduce the need for costly capital expenditures. With loaned instruments and implants accounting for up to 80 percent of the total cost of orthopedic surgery and the fact that SP professionals are largely responsible for receiving and managing loaned instruments, communicating needs and pertinent updates, and then reprocessing the sets before and after the procedure, it is vital that the SPD has a prominent seat at the table when conversations and policies surrounding loaned instruments take place. Having the right data surrounding loaned instruments—including statistics and information regarding late drop-offs/arrivals and late post-procedure pickups and the reasons behind them, and the ready availability of IFU—can generate necessary improvements and change and help SP professionals gain the essential resources needed in their departments, Berg said.
Loaned instrument data can be tracked and attained through instrument tracking software/programs, well-managed manual processes, or both (manual approaches require more time dedicated to the process, however). Regardless of whether a facility uses a manual or automated approach, a commitment to establishing an effective loaned instrumentation program will positively impact compliance, device traceability and accountability, Berg explained. Note: Facilities seeking guidance on how best to draft an effective management process and policy for loaned instruments can rely on AAMI Technical Information Report (TIR) 63, Management of loaned critical and semi-critical devices that require sterilization or high-level disinfection.
It is also necessary to build into the facility’s loaned instrument policy key elements to ensure accountability, compliance and safety for all parties involved. The policy should clearly outline requirements and expectations, and facilities should ensure vendors and all other key stakeholders receive a copy of the policy and sign off that they read the content and agree to the terms. Time requirements associated with loaned instrument drop-off must be established upfront and included in the written policy. Although SP professionals are not often involved in the earliest stage (the request for the loaned devices), they must still be aware of the details to ensure the SPD can safely and effectively meet the need within an adequate timeframe. It all comes down to what’s requested, says Berg. If a surgeon is performing a total knee procedure, for example, Company X will bring in a certain amount of trays for the case. In Berg’s previous facility role, he said that if the instruments were needed in the OR by Tuesday at 7 a.m., the SPD would need those total knee trays in the department by Monday at 7 a.m. because facility policy stated that instruments must arrive at least 24 hours before the procedure. If a drop-off occurred last-minute or at any point under 24 hours, that incident was documented (with the time of actual delivery and the reason for the late arrival). Some late deliveries are no fault of the vendor/distributor, Berg acknowledges, and are often due to an add-on procedure or other late request from the OR. Even so, recording those details is vital for recognizing trends and communication or compliance gaps, which can then be proactively addressed.
“If loaned instruments frequently arrive late despite advance notice from the OR, the SPD can take the data directly to the vendor and re-educate them on the policy and then work together to prevent future occurrences,” he noted, adding that surveyors will look to see what the facility’s loaned instrumentation says and review documentation of when the SPD receives loaned instruments. “If you have a 24-hour or 48-hour policy on when loaned instruments should be arriving in your facility [in advance of the procedure] and surveyors discover that policy is not being followed, you’ll be in violation of your own policy.”
Weight limits for loaned instrument sets should also be outlined in the policy. The Association for the Advancement of Medical Instrumentation (AAMI) recommends that instrument sets and trays prepared for sterilization not exceed 25 pounds (the weight of the container that houses the instruments is included in the 25-pound limit). Nonetheless, it is not uncommon for loaned sets to arrive that exceed that weight limit (sometimes, by only a couple pounds but, often, by significantly more). Working with the vendor to determine how best to reduce the set weight (perhaps by splitting the contents into two trays or working with the OR to determine whether any routinely unused instruments in the set could be removed from the tray and packaged separately in the event they are needed) is an effective strategy.
Ready access to current IFU is essential for ensuring SP professionals have the right information to manage and process all instrumentation safely and effectively, including loaned instruments. This IFU requirement should be expressly addressed in the loaned instrument policy, and SP professionals should verbally reiterate this need with their vendors. Before the procedure and the delivery of any loaned devices, SP professionals must also be able to verify they have the right equipment, supplies, education/IFU and other necessary resources to handle and reprocess those devices safely and properly. According to Berg, not all instruments and cycles (e.g., wash, sonic, sterilization) are created equally; therefore, SP technicians must have a clear understanding of which cycles are needed, and they should also have some knowledge of how the device is used. Further, they must know how instruments are disassembled, so they can be cleaned properly when they arrive to the facility (and again after being used in the procedure).
Instruments in loaned sets should be carefully inventoried upon receipt and when the vendor picks them up after use, and those contents should be documented. A manual or electronic count sheet that is readily available at the location where loaned instruments arrive and depart the facility helps SP professionals track set contents, and the list/count sheet should not be signed or otherwise approved without visual confirmation that all contents are not only in place but also not damaged. Taking photos of the set contents is wise for tracking and documenting all instruments upon arrival to and departure from the facility.
“If an instrument turns up missing or damaged after leaving your facility, imagine the benefit of being able to visually show they were present and undamaged at the time of pickup,” Berg explains.
Even with the best policies in place there will be times when rules aren’t followed, and the policy needs to be recommunicated. Berg recommends SP professionals work with their Infection Prevention partners, OR clinicians, and Materials Management/Purchasing partners to ensure all parties understand the policy and are held accountable when deviations occur. Circumstances will sometimes arise that result in loaned instruments arriving late, for example, and Berg stressed the importance of strong communication when such incidents arise. In his previous roles, when he knew the SPD would receive loaned instruments on Friday for a Monday procedure, for example, he would schedule more help in the department for Friday to avoid leaving all the work for the Saturday shift.
“It is critical to give enough time to check in the instruments, inspect them and make sure you have the IFU and everything else needed to process them properly,” he said. “Loaned instruments need to be viewed just like instruments in your facility’s inventory, with proper controls and care being given to them when they’re [in your possession].”
Julie E. Williamson serves as editor and communications director for the Healthcare Sterile Processing Association (formerly the International Association of Healthcare Central Service Materiel Management).
Sterile Storage Could be Compromising Patient Care, Safety
By David Taylor, MSN, RN, CNOR
Editor's note: This column originally appeared in the December 2021 issue of Healthcare Hygiene magazine.
Many factors need to be considered regarding sterile storage of instrumentation and supplies. Although sterile storage is a critically important aspect of sterile processing (SP), many SP professionals do not understand how the environmental controls that govern this process can dramatically affect patient safety and negatively impact the organization’s bottom line.
When developing infection prevention-related policies and practices for storage and supply areas, it is important that SP leaders not only know the infection prevention policies established by their institution, but also understand standards established by the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), which can vary depending on the date the storage space was established.
The first step SP leaders should take is ensuring their organization is compliant with all building code requirements. Once this has been established, the next step is to satisfy the Centers for Medicare and Medicaid Services (CMS) requirements for ventilation. Note: Criteria for new or renovated spaces (versus existing space) may vary. Contacting the healthcare organization’s Facilities team should be sufficient to determine which standards must be met. Keep in mind, however, that industry standards and local building codes can vary, and one’s organization will need to meet the most restrictive of the two.
Supply storage areas that support SP’s mission must follow all building codes and satisfy CMS ventilation requirements, which include the following:1
• Positive air pressure relationship to adjacent areas
• Minimum outdoor air exchange (two per hour)
• Minimum total air exchange (four per hour)
• Maximum relative humidity (60 percent)
• Temperature range (71-78 degrees F; 22-26 degrees C)
Although this is not an exhaustive list, other things to consider regarding SP supply storage include:
• All patient care supplies should be stored away from the edge of a sink to avoid possible splash contamination.
• All patient-care supplies must be at least five inches from the floor. If fixed or mobile wired shelving is used, ensure the lowest level shelf is covered with a solid surface (plastic cover) to avoid possible splash contamination during cleaning.
• Storage must maintain an 18-inch clearance from the ceiling (or the sprinkler head if co-located) to allow for the proper function of the fire and safety sprinkler system. It is important to know that the 18-inche rule applies laterally, away from any sprinkler heads as well.
• Corrugated cardboard material delivered to healthcare areas should be unpacked and broken down and removed in a timely manner. Use of corrugated cardboard cartons in healthcare storage areas must be assessed by the healthcare epidemiology team to ensure that the use of such cartons does not compromise the cleanliness or sterility of patient supplies.
• Internal shipping boxes (not corrugated boxes) may be used to dispense sterile and clean supplies but should be discarded when the box has been emptied.
• Doors should remain closed to maintain temperature, humidity, air flow (positive) and air exchange compliance.
• Wooden pallets should be limited to the transportation use by logistics and delivery personnel. Only pallets made of synthetic material are allowed in storage rooms where patient care supplies are located.
Once items are sterile, care and extreme caution must be consistently taken to prevent them from becoming contaminated. Sterile packages must be protected by keeping items in a safe environment and ensuring all staff members engage in proper, standards-based practices for handling and storage.2
David Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and the principal of Resolute Advisory Group LLC, in San Antonio, Texas. He has served as an IAHCSMM contributing author since 2019.
References:
1. The Joint Commission. Temperature and Humidity Requirements—Guidance for Storage or Sterile Supplies. 2019. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/environment-of-care-ec/000001275/
2. International Association of Healthcare Central Service Materiel Management. 2016. Central Service Technical Manual. 8th Ed., pp. 341-356.
Sterile Processing Certification Legislation Updates: Current Status, 2022 Plans
By Julie E. Williamson
This column originally appeared in the November 2021 issue of Healthcare Hygiene magazine.
Sterile processing (SP) functions involve challenging work, focus, attention to detail, and acquired knowledge and skill sets to ensure each task is performed safely, effectively, and in line with best practices, standards/regulations, and manufacturers’ instructions for use. Healthcare organizations that prioritize certification for their SP professionals will help ensure improved service quality, patient safety and infection prevention.
The International Association of Healthcare Central Service Materiel Management (IAHCSMM)’s top legislative priority is ensuring certification of all SP technicians across the country. Connecticut, New Jersey, New York, Tennessee and Pennsylvania are currently the sole states to require certification of SP technicians; however, IAHCSMM’s Government Affairs Director and Advocacy Committee are working hard to get certification legislation passed in other states.
In 2020, Pennsylvania became the fifth state to require certification of SP technicians. Getting the bill across the legislative finish line was a five-year process and a big win for the profession considering the pandemic-related changes that impacted states legislatures. Pennsylvania state legislators were the only individuals allowed in the building for a period, and similar precautions were seen in other states. Testimonies were held remotely and some legislative sessions, such as those in Rhode Island, took place with legislators shielded by plastic partitions, explained Josephine (Jo) Colacci, Esq., IAHCSMM’s director of government affairs, during her Oct. 22 session at IAHCSMM’s 2021 Virtual Conference & Expo.
“We didn’t do as much in 2021 because of COVID-19. I was concerned how it was going to impact legislatures and a lot of state budgets got hit, too, so we pulled back a bit on our status for 2021,” she said.
In Colorado, IAHCSMM planned to reintroduce its legislation in 2021. Although the certification bill had passed the state Senate and the House Committee, Colorado’s legislators recessed in 2020 due to the pandemic. When they reconvened, only a couple weeks remained in the year. Colacci learned that the governor would potentially veto the legislation, so the remainder of 2020 and into 2021 saw IAHCSMM reworking the bill. When the governor’s office still indicated the governor would veto the legislation, Colacci decided not to reintroduce it at that time.
“There’s supposed to be a large piece of legislation introduced in January 2022 that is going to change how Colorado regulates professions. When that happens, we’ll then reintroduce our legislation.”
In Massachusetts, where IAHCSMM first introduced a late-file bill in 2014, the process has been slow. The certification legislation passed the state Senate twice and made it to the final reading in the House in 2016; however, progress stalled thereafter. Fortunately, IAHCSMM’s testimony during an Oct. 15, 2021, committee hearing with the Joint Public Health Committee proved promising.
“I gave them the history that we passed out of their committee three times,” Colacci said, adding that the committee chairwoman acknowledged that the wait had been long and thanked Colacci for continuing to bring the legislation and its importance to their attention. Massachusetts has a two-year legislative cycle that ends in December 2022, and Colacci is hopeful the bill will be signed into law before then.
In Florida, a bill is being drawn up and will be introduced as soon as the state legislature convenes in March 2022. Florida’s legislative sessions last only two months, concluding in April 2022. IAHCSMM is working with a lobbyist in Florida, and a sponsor for the bill has already been identified.
“We’re hoping all this prework will bode well for us when this bill is introduced,” said Colacci. Still, she reminds that whenever a new bill is introduced in a state, organizations tend to come forward to comment and, at times, challenge some bill language, which can slow progress.
“There’s a good change our bill will not pass in its first year, but at least we’ll be introducing it, so we’ll know which issues arise and we can redraft and reintroduce the bill for 2023.”
Colacci shared an exciting development at the federal level as well—legislation that would allow for COVID-19 hazard pay (for work done in 2020 and 2021). Currently, the legislation applies to a long list of professionals, including surgical assistants and surgical technicians, and IAHCSMM hired a lobbyist with the Association of Surgical Technologists to get sterile processors included in the legislation. The federal lobbyist’s first step will be arranging meetings between Colacci and some sub-committee members, so Colacci can explain why sterile processors should be included in that piece of legislation.
“Once we get that, and I believe we will, we will then ask them to move the legislation.” She cautioned that this piece of legislation may not move as a standalone bill. Smaller pieces of legislation often get packed into larger bill packages, and she expects that will happen to move this legislation. She urged IAHCSMM members to watch their emails for action alerts that will ask members to contact their federal elected officials (a simple process that requires little more than a couple keyboard clicks and an electronic signature). “This bill will be alive until December 2022, and we’ll need many people contacting their federal legislators to try and get them to push this bill forward.”
Julie E. Williamson is director of communications for the International Association of Healthcare Central Service Materiel Management (IAHCSMM).
Infection Prevention, Patient Safety Hinge on Multi-Department Collaboration
By Tony Thurmond, CRCST, CIS, CHL, FCS
This column originally appeared in the October 2021 issue of Healthcare Hygiene magazine.
Healthcare organizations and each of their departments aren’t successful because of a single idea or individual effort. In fact, the best results in terms of efficiency, efficacy and safety come from collaboration and ongoing interdisciplinary teamwork, with all members working toward a common goal of infection prevention and other positive outcomes.
Often departments -- or even individuals within those departments -- aim to complete tasks on their own—either failing to seek assistance from others due to a fear of relinquishing control or concerns of conveying inadequacy regarding the task at hand. Some may also avoid seeking input or help from others because they consider themselves the experts and don’t want others interfering with the way things are done. Whatever the reason, any actions that inhibit teamwork and collaboration can lead to negative outcomes for employees, their department, their customers and, of course, the patients on the receiving end of the services. Any sterile processing (SP) professionals who aren’t working collaboratively with others are missing out on opportunities for growth, improvement and long-term success.
Positive changes in sterile processing departments (SPDs) and other healthcare service areas often come from reaching out to allied department (or, in many cases, multiple departments) and putting collective minds together to drive best results. Many departments can help the SPD reach its peak performance, including the following:
Surgical Services: Many collaborative opportunities await between the SPD and the operating room (OR), and those opportunities often lie within some of the common challenges that exist for each department. While those challenges can lead to finger pointing between the two departments, it is important to remember that all SD and OR employees are on the same team and seek the same outcomes: quality service, infection prevention and safe patient care. Designating a champion in the OR who will work with the SPD (and assigning an SPD liaison to the OR) is a great way to improve quality teamwork. When looking to improve point-of-use instrument care at a previous facility where I worked, I recruited the help of an assistant manager in the OR. I shared examples of how instruments arrived in decontamination in disarray and explained how they were dangerous and difficult for our technicians to handle. I also showed photos of heavy items placed on top of delicate equipment and instruments and provided examples of soiled instruments with dried-on soil, which created a valuable teaching moment regarding the dangers of bioburden and how much more difficult it is to clean when allowed to dry and harden on devices. I then reminded them how point-of-use care is a practice recommended by the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative Registered Nurses (AORN) and the Association of Surgical Technologists (AST); this provided documented proof of what is universally recommended and expected.
The outcome of these teaching moments was a collaborative effort to educate all OR staff to begin treating instruments at the point of use (moistening soiled instruments with an approved spray prior to their being sent to the decontamination area). We also began monitoring trays sent to decontamination and recorded in our instrument tracking system whether they were sprayed/properly moistened. Our compliance rate jumped from 15 percent to more than 64 percent in just over five months.
To further advance our commitment to collaboration, we invited our SPD staff to witness a surgical procedure. This allowed our technicians to see firsthand how the instruments are used and how the OR team expects to receive the devices to provide safe and on-time patient care. One of our SPD technicians shared that the experience in the surgical suite helped her learn the importance of having the right instruments in the tray (and all instruments arriving to the OR clean, sterile and well-functioning).
Infection Prevention: SP professionals must understand that most IPs are eager to learn as much as they can from those in the SPD. It’s also worth noting that accreditation organizations will look to IPs to ensure they have a full understanding of the facility’s sterilization practices. SP professionals should ask IPs to come and work with or observe in the department for a day or two. Most IPs will be eager to learn the processes and serve as another set of eyes on SP practices, and their involvement could shed more light on process improvement opportunities. Working closely with IPs can also help ensure that SP has the tools and resources needed to work safety, effectively and in accordance with standards and guidelines.
Biomedical Engineering/equipment vendors: Collaborating with biomedical engineers and equipment vendors allows them to see how SP equipment is being used. At a previous facility where I worked, we were visited by both Biomedical Engineering and equipment vendors; during that visit, it was identified how an ultrasonic could be moved closer to a workstation to save steps and help deliver optimal results. This collaboration also helped us identify high-use equipment and rotate equipment cycles for improved utilization.
Environmental Services: Partnering more closely with environmental services (EVS) professionals can help the SPD determine the most appropriate cleaning schedule. If EVS does not know the SPD’s needs or understand the reasoning behind them, the service may not be viewed as a top priority. Working together builds a stronger relationship that helps sets proper expectations to improve patient and employee safety.
Units/Clinics: Point-of-use instrument care is not always taught or demonstrated, so employees from user departments or clinics may lack the information and tools needed to complete the important function properly and consistently. They may even falsely assume instrument care is the sole responsibility of the SPD. One example is when flexible bronchoscopes or other endoscopes are brought to the floors/units for procedures. Endoscopy professionals don’t always understand they have a responsibility for point-of-use treatment of the endoscopes following their use. If this point-of-care step is skipped, most endoscope instructions for use call for an extended cleaning process, which can add many more hours to the reprocessing of the device. Effective collaboration and education about the importance of point-of-use instrument care will help eliminate these types of challenges.
C-Suite: Hospital administrators don’t always know the needs and challenges of the SPD such as insufficient staffing, limited instrumentation inventories, outdated or malfunctioning equipment, and lack of current industry standards, to name a few. SPD leaders must communicate clearly and regularly with those at the administrative level. The more those in the C-Suite know about the vital needs of the SPD, the more willing they will be to meet those needs and recognize the value of the department.
Working in siloes will rarely deliver positive outcomes, especially in a healthcare environment where all departments play a critical and often connected role in delivering exemplary services in the name of patient care, employee safety, and infection prevention. The open pursuit of interdisciplinary collaboration will help bring success to the organization and the individual departments by encouraging open communication to discuss unique challenges and practical solutions.
Tony Thurmond, CRCST, CIS, CHL, FCS, is an IAHCSMM past-president and IAHCSMM fellow who serves as central service manager at Dayton Children’s Hospital.
Dental Reprocessing: Guideline, IFU Adherence Vital to Infection Prevention
By David L. Taylor, MSN, RN, CNOR
Editor's note: This column originally appeared in the September issue of Healthcare Hygiene magazine.
Sterile processing (SP) is the first link in the chain of infection prevention. Its role is to decontaminate, clean, inspect and sterilize instrumentation for future use; however, failure to do so can introduce pathogens into the clinical environment and increase the risk for infection.
Safe, thorough device reprocessing isn’t just critical in hospitals; it’s also vital across all environments of patient care, including dental clinics. Understanding the process of infection transmission and prevention helps ensure sterile devices and a safe work environment for patients and healthcare professionals, including dental healthcare providers (DHCP). Because SP processes are often overlooked and misunderstood, ECRI identified “infection risks from sterile processing errors in medical and dental offices” as No. 3 on its list of Top 10 Health Technology Hazards for 2020.1 The American Dental Association (ADA), in collaboration with the Centers for Disease Control and Prevention (CDC), have updated and supplemented their infection control recommendations since 1993 to not only reflect new scientific knowledge but to grow the understanding of the principles of infection control practices.
Historical perspective
The 2016 CDC document, CDC Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care,2 brought together recommendations from the CDC 2003 guidelines on infection prevention3 , along with tools and checklists to help DHCP follow infection prevention guidelines.
The ADA urges all practicing dentists, dental auxiliaries and dental laboratories to employ appropriate infection control procedures as described in the 2003 CDC guidelines, and the 2016 CDC summary. Additionally, the ADA recommends that all settings keep up to date as new scientific information and regulatory guidelines leads to improvements in infection control, risk assessment, and disease management in oral healthcare.
Along with the proper sterilization of instruments and materials, sterilizer monitoring is an essential part of any in-office/clinic infection prevention program. The CDC advises that dentists use only FDA-cleared dental handpieces and sterilize them in accordance with manufacturers' instructions for use (IFU). Following manufacturers’ instructions for sterilization and processing of all dental instruments and materials is critical to patient and employee safety. Sterilizer monitoring and efficacy testing is an essential part of this process.
Each step of instrument processing must be completed per IFU, accepted guidelines and recommendations—with no steps missed or altered in any way. DHCP who clean, inspect, package, sterilize and store instruments must have the skill sets and knowledge of each process and understand the science behind each step. Unfortunately, DHCP often have a myriad of other clinical responsibilities that can divide their attention. As a result, failure to consistently and effectively clean and disinfect or sterilize contaminated items before reuse can expose patients and DHCP to virulent pathogens and create a significant risk of disease transmission within the dental setting. Between 2003 to 2015, disease transmissions in dental settings, including patient-to-patient transmissions, has been well documented.4-7
Inconsistency in knowledge
The question that must be asked is whether all employees responsible for SP functions have adequate knowledge to mitigate errors and consistently deliver safe, well-functioning instruments and services to meet their customers’ needs. Unfortunately, in my personal experience as a consultant, the answer is often no.
With the complexities inherent to SP functions—and inconsistencies in design, equipment, material and processes found in dental practices and clinics—careful attention must be paid to ensure all employees are trained to the same standard and have the knowledge necessary to manage their SP duties safely and effectively.
Instrument processing areas
The instrument processing area should be centrally located within a healthcare facility and have a one-way/dirty-to-clean flow to reduce the possibility of cross contamination between dirty and sterile devices. In dental facilities, instrument processing can be located/performed in one space as long as there is a linear flow, without the risk for crossover between dirty and clean.
The instrument processing area includes the following four distinct areas, regardless of whether it is located in dental or traditional medical settings:
1. Receiving, cleaning and decontamination (three-sink system for hospital/medical settings)
2. Preparation and packaging
3. Sterilization
4. Storage
As a result of design, clinic acquisition or other factors, instrument processing areas can vary in size and can compromise DHCP’s ability to remain consistent with their processes and infection prevention strategies. Some dental SP areas are so small that DHCP have to create work arounds to complete certain tasks.
Water quality
Water quality is a concern in dental practices across the country. Water quality is critical for the sterilization process, and poor water quality can impact the sterilizer’s ability to function properly. Poor water quality can also damage and reduce the life expectancy of instruments and processing equipment. Critical water, such as distilled, is the recommended water type for use in countertop sterilizers; however, many dental practice clinics use various water filtration systems—with no assurance that these systems meet the recommended water purity standards.
It is highly recommended that water testing be conducted for all water sources to ensure it meets the water purity specifications or parameters outlined in the IFU. Note: If a filtration system meets all parameters for use, dental facilities should consider standardizing filters across their practice or clinic system, while also developing water testing methods, establishing testing frequency, and documenting the same going forward. If filtration systems do not meet water purity standards, it is essential that those filters are removed from use and that distilled water is provided.
Biological testing and instrument tracking
Biological indicator (BI) vials contain Geobacillus stearothermophilus bacterial spores and are intended for monitoring the efficacy of steam sterilization. Biological monitoring allows facilities to take control of their steam sterilizer testing and provides peace of mind that the sterilizer is functioning properly. It also helps organizations follow CDC guidelines, which recommend that steam sterilizers be monitored at least weekly. Although the CDC recommends weekly BI testing (at a minimum), the prefer method is to perform BI testing daily, as well as with every load that contains implantable devices. Dental SP professionals across the country have come under greater scrutiny by regulatory and government agencies; therefore, BI testing on every load is highly recommended (Note: BI monitoring for each load is not included in the standards; however, many facilities have gone to a higher level by performing BI testing for each load).
Instrument tracking
Instruments that are reprocessed and rendered safe for patient use should be traceable; however, in the dental environment, few offices or clinics can effectively track their instrumentation to a sterilization cycle. Once sterilized, those instruments are placed on trays, in bins and/or in cabinets for future use. In some cases, they are used immediately following sterilization.
If a BI test comes back positive, it is important that dental professionals have a way to track instruments and when they were reprocessed. If a positive BI result comes back from the lab and instruments from that particular day or subsequent days were used, the results could be catastrophic.
Individual instruments and sets should be logged for every sterilizer load. This allows organizations to track when instruments were processed, under what conditions, and whether parameters were appropriately documented. If a recall is needed (based on a positive BI test result or equipment malfunction, for example), instruments should be able to be located easily, in real time. Documentation can be as simple as a logbook or use of a record envelope. Some institutions and groups use a patient tracking card and document load specifics in the patient’s record.
Currently, The Joint Commission (TJC) is asking operating room (OR) and SP professionals across the country how they track their instruments to the patient (e.g., which instruments were used on which patients and when?). The surveyors are then retracing each step in the process back to SP and validating that all processes were met in the reprocessing of those items. All documentation is being reviewed to include (but not be limited to) date, time, parameters met or unmet, staff member(s) involved, and how those instruments are being stored (temperature and humidity). For the staff member who processed those instruments, TJC surveyors will review that individual’s competency, training and education. Note: TJC and the Centers for Medicare and Medicaid Services (CMS) are now sharing findings with one another. Depending on the severity of the TJC’s findings, CMS may initiate its own investigation and survey the organization/facility. Again, this is important because dental industry practices are coming under greater scrutiny these days.
Ultrasonic equipment cleaning and efficacy testing
Proper cleaning is the first step to effective instrument reprocessing. If debris (bioburden) is not removed, the instrument cannot be effectively sterilized. Automated processes such as ultrasonic cleaning aids in the proper cleaning of instruments. Ultrasonic cleaners are used to remove soil from difficult-to-clean areas such as joints and crevices, using cavitation. Cavitation is a process in which low-pressure bubbles in a cleaning solution burst inward and dislodge soil from instruments. Ongoing routine testing of equipment used to reprocess instrumentation is necessary to ensure each piece of equipment is functioning properly and as intended.
During my observations as a consultant, dental staff often report they are changing the ultrasonic fluid and using the correct type and amount of detergent (per IFU) twice a day or “as needed” as water becomes cloudy; however, when asked, many dental professionals could not answer how many individual instruments or cassettes may be processed before each water change or how long it took before water became cloudy. In addition, most dental offices and clinics do not keep logs showing when the units were cleaned, and/or they fail to document in-between water changes. In addition, cloudy water can be a subjective observation; therefore, leaving it (and the need for water change) up to the user’s interpretation is risky. If time pressures exist (which is not unlikely), dental staff may forgo a water change until the next batch of instruments—a decision that could jeopardize patient safety. ANSI/AAMI ST79, Section 7.6.4.4, states that when using an ultrasonic cleaner, each load should use a “fresh cleaning solution,” which includes detergent. Each time the sonic is filled, it should also be degassed. Further, the sonic should be cleaned each day in accordance with the IFU.
To ensure equipment is working properly, efficacy testing should be done routinely, and the results should be documented, per ANSI/AAMI ST79, Section 13.2. Ultrasonic machines are frequently used in dental practice. In my experience, many dental employees are unaware of what efficacy testing is and what it entails. When asked what the test results should look like when using that product, different answers are common (dented, pin hole, etc.). Also, when asked how they test the machine’s effectiveness, many state that they perform an aluminum foil test. Foil tests, unfortunately, do not ensure proper function of the ultrasonic device. If three different manufacturers of foil products were used for this type of testing, each test would be subjective and left up to the determination of the user, which is problematic.
Efficacy testing must be done daily, with the appropriate challenge test to ensure each unit performs cavitation and can remove soil. It is highly recommended that dental organizations incorporate appropriate monitoring of their ultrasonic machines and document testing results. There are commercial monitoring tests available that monitor cavitation, and a test that contains a test soil that mimics the presence of blood and tissue that may be found on an instrument after use. During the test, the monitoring strip assesses multiple variables of the cleaning process, including cavitation, time, temperature and detergent. The IFU of the test should be reviewed for what, specifically, it tests. Full removal of the test soil from the strip indicates an effective cleaning process and provides greater assurance that the equipment is functioning properly.
Steam sterilization integrator
Many dental practices forgo the use on an integrator and rely on a color dot located on peel packs as the final result. Type 5 multi-variable sterilization monitor integrators are designed to react to all critical variables in the sterilization cycle (time, temperature and the presence of steam). Indicator strips should be used in each peel pack and cassette that will undergo reprocessing.
Type 5 integrators that utilize a moving front indicator technology (an advancing color bar that moves from “reject” to “accept,” for example, and eliminates the need to interpret or match colors) are highly recommended. Because staff knowledge varies greatly, it is also recommended that an educational fact sheet be displayed in all SP areas to clearly identify an item that should be rejected and one that meets acceptable sterilization parameters.
Instrument packaging
Peel Packs/Pouches Versus Cassettes
The CDC guidelines state that items to be sterilized should be arranged in such a way to permit free circulation of the sterilizing agent (e.g., steam, chemical vapor, or dry heat).8,9 An instrument peel pack that is overfilled may compromise sterilization. In addition, overfilled peel packs have and increased risk of an instrument poking through the packaging, resulting in contamination and a greater likelihood of sharps injury.
It many dental practices, DHCP have a choice in the method in which they package their instruments. Some primarily use cassettes; however, some locations are instead opting for peel pack/pouches. For those that use peel pack/pouches, it is important to ensure instruments are placed properly and not overly crowded to ensure sterilant (steam) comes in contact with all instrument surfaces. The preferred orientation for sterilization peel pack/pouches is to place peel packs/pouches on their edge.
Note: When cooling hot instrumentation, the packages should remain in the basket or tray they were sterilized in to avoid touching the packages as they are removed from the sterilizer. Many DHCPs place items on an adjacent flat surface. The surface temperature of these areas can vary greatly from the instruments being removed from the sterilizer, which can cause condensation and result in contaminated instruments. An infrared device can be used to measure the temperature of the package before it is handled.
Conclusion
To reduce the risks associated with instrument reprocessing, governmental agencies and other professional associations have created recommendations, guidance and regulations that help healthcare organizations, including dental practices, to maintain safe environments for the patients they care for and the staff members who work in these areas. Due to its impact on patient outcomes and infections, the SP function is facing greater scrutiny. Device reprocessing has several necessary steps that must be done correctly every time in order to render devices safe and ready for use on subsequent patients. Monitoring the sterilization process must be done and documented appropriately to show compliance with current standards, guidelines, regulations and IFU. Not knowing is not an excuse and has caused organizations to fail inspections, suffer bad publicity from negative outcomes resulting from failed SP processes—and above all, negatively impact patient and employee safety.
Instrument processing is the most important component of an infection prevention program. It is a complex process that requires specialized equipment, adequate space, qualified personnel, and routine monitoring for quality assurance. As dental and medical personnel process instruments for safe patient care, it is imperative that they receive adequate and ongoing education and training from credible sources regarding the entire instrument processing practice, including but not limited to transport, cleaning, packaging, sterilization and storage.
Guidelines from the CDC continue to change and are the standard for infection control in dental settings. Clinicians should also consult the manufacturers’ IFU for reprocessing dental and medical instruments for each reusable instrument or device within service. Note: Other resources available include the Organization for Safety, Asepsis and Prevention, which offers an extensive online collection of resources, publications, FAQs, checklists, and toolkits to help dental professionals deliver the safest dental visit possible for their patients.10
David L. Taylor, MSN, RN, CNOR, is the principal of Resolute Advisory Group LLC, a healthcare consulting firm in San Antonio, Texas. He has served as an IAHCSMM author since 2019. For more information, email David@ResoluteAdvisoryGroup.com
References:
1. Special Report Top 10 Health Technology Hazards for 2020, Expert Insights from Health
Devices https://elautoclave.files.wordpress.com/2019/10/ecri-top-10-technology-hazards-2020.pdf
2. https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf
3. https://www.cdc.gov/mmwr/PDF/rr/rr5217.pdf
4. Redd JT, Baumbach J, Kohn W, et al. Patient-to-patient transmission of hepatitis B virus
associated with oral surgery. J Infect Dis. 2007;195(9):1311–1314.
5. Radcliffe RA, Bixler D, Moorman A, et al. Hepatitis B virus transmissions associated with a portable dental clinic, West Virginia, 2009. J Am Dent Assoc. 2013;144(10):1110–1118.
6. Oklahoma State Department of Health. Dental Healthcare-Associated Transmission of
Hepatitis C: Final Report of Public Health Investigation and Response, 2013. Available at: http://www.ok.gov/health2/documents/ Dental%20Healthcare_Final%20Report_2_17_15.pdf.
7. Klevens RM, Moorman AC. Hepatitis C virus: An overview for dental health care
providers. J Am Dent Assoc.2013;144(12):1340–1347.
8. Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Centers for
Disease Control and Prevention. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
9. Centers for Disease Control and Prevention. Summary of Infection Prevention Practices
in Dental Settings: Basic Expectations for Safe Care.
https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf. October 2016.
10. Organization for Safety, Asepsis and Prevention website. www.osap.org
Sterile Processing Week is October 10-16: It’s Not Too Early to Get Your IPs and C-Suite Involved
By Julie E. Williamson
Editor's note: This column originally appeared in the August 2021 issue of Healthcare Hygiene magazine.
Getting other disciplines involved in Sterile Processing Week events isn’t just good for sterile processing (SP) employee morale; it can also promote better communication, partnerships and support, long after the dedicated week off honor concludes.
While it’s important to invite surgical services members (and other departments the SPD routinely serves) to planned events, it’s essential to not overlook the facility’s infection preventionists (IPs) and administrators. IPs can be some of SP professionals’ greatest sources of support, and both sides can benefit significantly from a successful long-term partnership. SP leaders who haven’t yet forged an effective relationship with their IP(s) are encouraged to make those introductions now. From there, they should include them in various Sterile Processing Week functions that might be planned throughout the dedicated week and work together to build a framework for a more productive interdisciplinary partnership.
Prior to the week’s official kickoff, set up a meeting with the IP(s) to discuss the SPD’s challenges, successes and goals, and then work together to help effectively address them. Is the SP team functioning with too few full-time employees? Is the department’s processing equipment breaking down more frequently? Is the SPD’s standards library out of date or is the SPD being pressured to rapidly turn around the same set of instruments because inventory doesn’t match the increasing case volume? Has the SP team expertly managed increased service demand with exceptionally low error rates? These are all critical points to address with the IP who can then help go to bat for the SPD—with data in hand—to the operating room, facility executives and other department heads, as needed, to help instill positive, standards-based change. This IP-SP alliance could even help expand the SPD’s capital budget to better meet the facility’s/departments’ critical needs.
SP leaders should also ask their IPs to help honor Sterile Processing Week and the SP team by writing a brief statement about the department’s critical importance to patient safety and quality care, and then sharing some impressive statistics (e.g., low error rates, number of sets processed for the year thus far, how the department greatly reduced immediate use steam sterilization rates; employee certification, and so on) in the facility’s newsletter or in the SPD’s own materials created for Sterile Processing Week. SP leaders can also ask their IP(s) to lead a Sterile Processing Week educational session. The opportunities are endless!
Those in SP leadership positions will also serve their team (and the facility) well by inviting the healthcare organization’s top-level executives to the department for quality education and a tour of all that takes place within the SPD’s walls. This invitation can prove valuable because some C-level executives lack a firm understanding of the importance of sterile processing and may not know the extent of SP professionals’ contributions, successes and sacrifices; therefore, it’s up to SP leaders to make them aware.
What might that look like during Sterile Processing Week? Try reaching out directly to hospital administration, surgeons and senior leadership from all other departments that utilize the SPD’s services in the facility. Invite them to observe the intricate workflow processes that must be followed to promote positive quality outcomes and patient safety—and be sure to explain these steps and processes along the way. Additionally, consider sharing the future goals and objectives that the SPD is working on that align with the healthcare organization’s mission, vision and values.
Bear in mind that many SP professionals had to endure process changes and create standard operating procedures during the pandemic that were/are out of normal operational business routines. This has likely led to valuable stakeholder meetings, which (hopefully) opened doors to improved communication and interdisciplinary collaboration. It’s up to each SP leader to ensure those doors stay open and that the department/discipline holds a prominent seat at the table, all year long.
Note: Sterile Processing Week happens to fall during the 2021 IAHCSMM Annual Conference & Expo, and IAHCSMM is excited to celebrate attendees and the collective profession throughout that week. What better way to celebrate than with IAHCSMM and so many of your peers and fellow industry experts? If you haven’t yet registered for this year’s Conference & Expo, don’t wait as standard registration rates end Sept. 24. For more information and to register, visit: www.iahcsmm.org/GreatExpectations.
IAHCSMM also provides useful resources to help you celebrate Sterile Processing Week in some meaningful, memorable and inspiring ways (visit iahcsmm.org/about/sp-week.html). IAHCSMM is currently updating its free Sterile Processing Week celebration templates (including cards, posters and employee appreciation certificates) that can be customized, downloaded, printed and shared. The templates will be updated and ready for downloading in September.
Julie E. Williamson is IAHCSMM’s director of communications/senior editor.
Spotlighting Best Practices and Infection Prevention
By Julie E. Williamson
Editor's note: This column originally appeared in the July 2021 issue of Healthcare Hygiene magazine.
Sterile Processing (SP) plays a vital role in care delivery, customer service, infection prevention and positive patient outcomes, so it’s imperative that SP professionals—who are responsible for cleaning, disinfecting, sterilizing, inspecting and delivering medical/surgical instruments to the operating room and other patient care areas—stay educated on best practices and the very latest standards and guidelines.
All who attend the 2021 IAHCSMM Annual Conference & Expo in October in Columbus, Ohio will have access to leading-edge education taught by the most knowledgeable and respected experts in the industry. They will leave the conference with new-found knowledge about the best practices to keep their departments running optimally and safely, and valuable steps to ensure the instruments they manage are properly cleaned, disinfected/sterilized and well-functioning.
The pandemic and its related uncertainties over the past year-and-a-half led IAHCSMM to move its Conference & Expo from May to October. IAHCSMM also embraced an innovative scheduling approach, with two back-to-back attendance options (Conference Option A takes place Oct. 9-11; Conference Option B takes place Oct. 12-14). Each conference option will have a different keynote speaker; however, all educational sessions and speakers will be identical for Option A and Option B. The two-day Expo will take place Oct. 11-12—on the final day of conference Option A and the first day of conference Option.
What follows is a summarized schedule of events for both conference options. A virtual conference option (Oct. 15-28) is also being offered for those unable to attend the in-person event. In-person attendees will receive complimentary access to the virtual conference.
Whichever option attendees choose, they can be confident they will receive the very best education and networking opportunities available to promote quality, safety and professionalism, and ensure they’re able to meet their healthcare customers’ and patients’ needs.
Julie E. Williamson serves as IAHCSMM’s senior editor and director of communications.
Option A Session and Event Schedule
Saturday, Oct. 9
10:30-11 a.m.: Opening Remarks
11 a.m.-12 p.m.: Opening Keynote (Steve Pemberton)
1:30-2 p.m.: Educational Session (Advocacy Update)
2:15-3:15 p.m.: Educational Session (Update on Sterile Processing Best Practices)
3:30-4 p.m.: Educational Session (Water Safety & Quality During Medical Device Reprocessing)
4:15-4:45 p.m.: Educational Session (Instrument Care & Handling: How to Care for Your Microsurgical Instruments in 5 Easy Steps)
5-6 p.m.: Educational Session (Is It Clean? Building a Quality & Audit Program Around Critical Elements Before Packaging)
7 p.m.: Opening Reception for Attendees
Sunday, Oct. 10
7:30 a.m.-8 a.m.: Educational Session (Infection Prevention 101)
8:15-8:45 a.m.: Educational Session (How to Evaluate Stains After Steam Sterilization)
9-10 a.m.: Educational Session (Are You Ready for the New AAMI ST91 for Endoscope Reprocessing?)
10:15-11:15 a.m.: Educational Session (Bringing Quality & Innnovation to Sterile Processing)
11:30 a.m.-12 p.m.: Educational Session (How to Tackle Steam Sterilization Failures)
1:30-2:30 p.m.: Educational Session (Sterile Processing Unscripted)
2:45-3:45 p.m.: Educational Session (Lessons from the Field: Preparing for the Next Pandemic)
4-5 p.m.: Educational Session (Fact or Facebook Fiction?)
5:15-5:45 p.m.: Educational Session (What You Should Know About the Physical Ergonomic Challenges Facing Sterile Processing Professionals)
Monday, Oct. 11
7:30-8 a.m.: Educational Session (Your Future Is in Your Hands: Building a Career Ladder in Sterile Processing)
8:15-8:45 p.m.: Educational Session (A Joint Commission Survey: What You Need to Know in Regard to High-Level Disinfection)
9-10 a.m.: Educational Session (Infection Control for the COVID-19 Era and Beyond)
10:15-11:15 a.m.: Educational Session (When Everything Goes Wrong: A Patient’s Perspective)
11:30 a.m.-12 p.m.: Educational Session (Understanding Enzymes and How to Get the Most out of Enzymatic Detergents)
1:30-2 p.m.: Educational Session (A Practical Guide for Disinfectants in SPD)
2:15-2:45 p.m.: Educational Session (Putting First Things First: What’s Your Priority?)
2-6:30 p.m.: Expo Open
Option B Session and Event Schedule
Tuesday, Oct. 12
10:30-11 a.m.: Opening Remarks
11 a.m.-12 p.m.: Opening Keynote (Jon Dorenbos)
1:30-2 p.m.: Educational Session (Instrument Care & Handling: How to Care for Your Microsurgical Instruments in 5 Easy Steps)
2:15-3:15 p.m.: Educational Session (Advocacy Update)
2-6 p.m.: Expo Open
6:30 p.m.: Evening Reception
Wednesday, Oct. 13
7:30-8 a.m.: Educational Session (Water Safety & Quality During Medical Device Reprocessing)
8:15-8:45 a.m.: Educational Session (Infection Prevention 101)
9-10 a.m.: Educational Session (Update on Sterile Processing Best Practices)
10:15-11:15 a.m.: Educational Session (Is It Clean? Building a Quality & Audit Program Around Critical Elements Before Packaging)
11:30 a.m.-12 p.m.: Educational Session (How to Evaluate Stains After Steam Sterilization)
1:30-2:30 p.m.: Educational Session (Are You Ready for the New AAMI ST91 for Endoscope Reprocessing)
2:45-3:45 p.m.: Educational Session (Bringing Quality & Innovation to SP)
4-5 p.m.: Educational Session (Sterile Processing Unscripted)
5:15-5:45 p.m.: Educational Session (What You Should Know About the Physical Ergonomic Challenges Facing Sterile Processing Professionals)
Thursday, Oct. 14
7:30-8 a.m.: Educational Session (Understanding Enzymes and How to Get the Most out of Enzymatic Detergents)
8:15-8:45 a.m.: Educational Session (A Practical Guide to Disinfectants in SPD)
9-10 a.m.: Educational Session (Lessons from the Field: Preparing for the Next Pandemic)
10:15-10:45 a.m.: Educational Session (A Joint Commission Survey: What You Need to Know in Regard to High-Level Disinfection)
11 a.m.-12 p.m.: Educational Session (When Everything Goes Wrong: The Patient’s Perspective)
1:30-2:30 p.m.: Educational Session (Infection Control for the COVID-19 Era and Beyond)
2:45-3:15 p.m.: Educational Session (The Future Is in Your Hands: Building a Career Ladder in Sterile Processing)
3:30-4:30 p.m.: Educational Session (Fact or Facebook Fiction)
4:45-5:45 p.m.: Educational Session (How to Tackle Steam Sterilization Failures)
6-6:30 p.m.: Educational Session (Putting First Things First: What’s Your Priority?)
7 p.m.: Closing Reception
To register for the 2021 IAHCSMM Annual Conference, visit: https://s6.goeshow.com/iahcsmm/annual/2021/index.cfm
Must-Have Standards for Every SPD: Value Letter Helps Ensure Budgets Allow for These Vital Resources
By Julie E. Williamson
Editor's note: This column originally appeared in the June 2021 issue of Healthcare Hygiene magazine.
Sterile processing departments (SPDs) operating in the absence of the latest industry standards, guidelines and recommended practices face the potential for numerous negative outcomes, including an increased risk to patient and healthcare worker safety, survey citations and fines, and devastating damage to their facility's reputation.
It's a message voiced by many experts—from sterile processing veterans, consultants, and surveyors from the Joint Commission (TJC) and Centers for Medicare and Medicaid Services (CMS), to litigators, representatives from healthcare associations and government agencies, and more. Unfortunately, many facilities lack these critical resources.
Sometimes, facilities don't have any version of certain standards; others may only have outdated versions – such as for ANSI/AAMI ST79, Comprehensive guide to steam sterilization and sterility assurance in health care facilities, which was updated in 2020.
Having current standards is not just vital for the SPD, but also for the collective facility. ANSI/AAMI ST79, for example, is a comprehensive document with valuable information for the SPD, operating room, infection prevention, safety, risk management, and engineering/facilities management, and it’s also the document by which surveyors are being trained.
Limited budgets are often to blame for the lack of standards in SPDs across the country. To help sterile processing professionals petition their facilities for the most current standards, guidelines and recommended practices needed for quality customer service and patient safety, IAHCSMM drafted the following Standards Value Letter, a template that sterile processing managers can customize and share with administrators and other executives.
We encourage any sterile processing manager who has struggled to attain the latest standards, guidelines and recommended practices to tailor this template to their own needs in an effort to drive best practices within their department—and we also recommend they lean on their organization’s infection prevention team for additional support, whenever needed, as infection preventionists can serve as a strong SPD ally.
Julie E. Williamson is editor/communications director for IAHCSMM.
Dear _____________________ (insert name of manager, administrative executive or other):
The Sterile Processing (SP) profession is ever evolving in terms of surgical instrumentation, processing equipment, and standard and best practices. To stay abreast of all these changes, it’s essential that our SPD and the professionals who comprise it have ready access to the most current standards and guidelines, such as those provided by the Association for the Advancement of Medical Instrumentation (ANSI/AAMI), the Association of periOperative Registered Nurses (AORN) and the Centers for Disease Control and Prevention (CDC).
Access to the latest standards helps ensure that our department is consistently following the most current recommendations and best practices that will promote patient and employee safety and keep us in check with The Joint Commission and Centers for Medicare & Medicaid surveyors (who are now being trained on standards such as ANSI/AAMI ST79: 2017 & 2020 Amendments A1, A2, A3, A4 (Consolidated Text), Comprehensive guide to steam sterilization and sterility assurance in healthcare facilities.
Industry experts recommend the following key resources for every SPD:
1. ANSI/AAMI ST79: 2017 & 2020 Amendments A1, A2, A3, A4 (Consolidated Text) Comprehensive guide to steam sterilization and sterility assurance in health care facilities
2. AORN Guidelines for Perioperative Practices, 2020
3. ANSI/AAMI ST58:2013, Chemical sterilization and high-level disinfection in health care facilities (Note: This document is currently under review)
4. ANSI/AAMI ST41: 2008/(R) 2018, Ethylene oxide sterilization in health care facilities (for facilities using ethylene oxide)
5. ANSI/AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities
6. ANSI/AAMI ST90:2017, Processing of health care products - Quality management systems for processing in health care facilities
7. AAMI TIR 68:2018, Low and intermediate-level disinfection in healthcare settings for medical devices and patient care equipment and sterile processing environmental surfaces
8. AAMI TIR 67:2018, Promoting safe practices pertaining to the use of sterilant and disinfectant chemical in health care facilities
9. AAMI TIR 34:2014, Water for the reprocessing of medical device
10. AAMI TIR 63:2014, Management of loaned critical and semi-critical medical devices that require sterilization or high-level disinfection
11. CDC Guideline for Decontamination and Sterilization in Healthcare Facilities, 2008
The CDC Guideline for Decontamination and Sterilization in Healthcare Facilities, 2008, is available free of charge on the agency’s website (https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf). Note: This version was updated in May 2019 and our SPD has this current version on file.
There is a charge for the other documents; however, I’m sure you will agree that budgeting for these documents is prudent given our healthcare organization’s desire to provide the very best patient care and quality customer service. Failure to have current standards on file in our department could prove costly for our facility if adverse events take place, such as surveyor citations/fines, costs associated with treating preventable infections or injuries to patients or staff, or litigation risks that could damage our facility’s reputation.
Diligent adherence to the latest standards and best practices will help drive patient and employee safety, advance infection prevention efforts, and keep exemplary customer service a top priority. It is my hope that you will support this request for budget dollars to allow for our department’s purchase of the most current standards as they become available.
Please let me know if you have any questions or concerns or require additional information. In the meantime, I’d like to thank you for your time and consideration.
Sincerely,
Competencies: Their Key Role in Sterile Processing Quality
By Tony Thurmond, CRCST, CIS, CHL
This column originally appeared in the May 2021 issue of Healthcare Hygiene magazine.
Competency is best defined as “a set of defined behaviors that provide structured guidance enabling the identification, evaluation and development of the behaviors in individual employees.” Put simply, it’s a term that relates to performance improvement, and professionals are expected to attain it through experience and an ability and desire to learn and adapt.
In the sterile processing (SP) realm, it’s important to examine how competencies are valued and applied. Are they viewed as a necessary evil or do SP professionals embrace the opportunities competencies can provide? ANSI/AAMI ST79, Section 4.2.2, states that “the responsibility of sterile processing should be assigned to qualified individuals who have demonstrated competence in all aspects of sterile processing.” Qualifications for SP professionals include demonstrated knowledge of and documented competencies in the tasks they perform, and a working knowledge of the work environment. Documentation is critical because if there must be evidence that proper training and demonstration of that training took place. Surveyors will ask to see employees’ orientation checklists and the documented competencies for each employee.
Develop a competency checklist
Thorough competency checklists must be created and maintained for each of the following areas of the SP department (SPD):
1. Decontamination: Competency must be shown in instrument sorting, disassembly/reassembly, manual and mechanical cleaning methods, microbicidal processes, equipment operation, standard- and transmission-based precautions, and engineering and work practice controls.
2. Instrumentation: SP professionals must know the names and descriptions of instruments in the organization’s inventory as well as the demonstrated inspection points of each device. Other competencies include proper preparation and packaging methods for sterilization.
3. Sterilization/High-level Disinfection (HLD): Competencies should cover all sterilization practices and principles, including steam, low-temperature, ethylene oxide, and HLD processes.
4. Worker Safety and Environmental Safety: SP professionals should understand and be able to demonstrate how to properly handle emergent situations, environmental hazards and other patient safety situations.
More competencies can be developed for specific equipment and instrumentation (to include proper cleaning techniques, sterilization and maintenance of each).
It’s important to note that a completed competency does not ensure a technician is competent, much the same way that holding certification does not ensure an individual’s competency. The proper skills learned and used effectively in day-to-day operations are what make for a competent technician. Some technicians can demonstrate the correct way of performing a task during a competency review, but then return to their bad habits after the review. Still, a competency checklist can benefit the department and facility in numerous ways, as the following paragraphs will demonstrate.
New employee checklists
When developing a competency checklist, equipment and departmental design will dictate the processes and workflow necessary to be completed. First, an orientation checklist must be developed and implemented for new SP employees. New team members should work with a competent technician who is willing to train and has the skills required to properly onboard the new employee. This orientation checklist is typically completed after 90 days of employment to verify the skills learned and the likelihood of the department continuing with the individual’s employment.
If the new employee demonstrates they have retained the training information provided and have the desire to continue to learn and broaden their skills, this growth should be documented by the SP leadership during the 90 days. If areas of weakness surface or it is determined certain information was not retained, the supervisor must review the training process and its effectiveness. The supervisor may need to review the preceptor or trainer to determine whether the proper information was given and whether certain aspects of the training process could be improved.
If no improvement is needed in the training process and the preceptor is providing proper, effective training, it must then be determined whether the new hire can be successfully trained and prepared for their role. Everyone learns at their own pace; however, the individual must demonstrate the desire to learn and do their best if they are to move past their 90-day review and continue to grow into a strong, proficient SP professional. It is recommended that new employees both verbalize and demonstrate the task(s) being evaluated. If, after completing the onboarding process and 90-day review, it is determined that the employee is not grasping the information and is showing little signs of succeeding in the role, it is best to inform this individual and not continue with their employment.
Be thorough and detail focused
Competency checklists must be written in the proper order of the process or workflow for the desired knowledge and skills being reviewed—and each item to be reviewed must be thoroughly understood and/or demonstrated. The checklist should indicate whether the demonstration is verbal or demonstrated, and it must be marked as “satisfactory” or “unsatisfactory” by the SP supervisor or manager. Each task/line item must be initialed by the person reviewing it to document that a thorough review was performed. Checklists should also include an area for comments (which can include suggested training or positive comments for technicians who demonstrated success and aptitude with a particular task).
Competency checklists must be reviewed at least once every six months to determine areas in need of improvement. Managers should ensure the checklist is current, taking into consideration any changes to equipment or internal processes, and whether any standards updates occurred that could require a change to current practices.
Most competency checklists should be completed at least once a year, or more often if a process is complex. If more errors have been identified in certain areas or for certain processes, an impromptu competency review may be necessary. Keeping a close eye on processes and employee training is necessary for promoting ongoing quality and safety.
Competency checklists may be developed from instructions for use of products or equipment, or from published standards such as those from the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative Registered Nurses (AORN) and others. Competency reviews of each technician must be reviewed by a competent technician, educator or manager who has also demonstrated working knowledge and expertise of the area being reviewed/assessed. The checklist must be signed by both the evaluator and the individual being evaluated. After the review, the competency must be placed in the employee’s file where it can be accessed for review as needed.
Competency checklists or reviews are considered a mandatory requirement in each department, and they allow departmental leaders to assess areas in need of improvement and gauge employees’ consistent adherence to best practices. Checklists can also lead to more standardization regarding how tasks and processes are performed, which can further lead to performance improvement and error reduction.
Examples of competency checklists are available free of charge to IAHCSMM members at https://www.iahcsmm.org/resource-documents/cs-sample-documents.html (members must log in to their member portal to access the documents). Another valuable resource is the IAHCSMM webinar “Understanding and Developing a Competency Program”(available until Sept. 30, 2021); members can view IAHCSMM’s webinars at no cost (non-member rate is $15).
Tony Thurmond, CRCST, CIS, CHL, is an IAHCSMM past-president and serves as central service manager at Dayton Children’s Hospital.
Peeling the Onion: How C-Suite Involvement, Consulting Can Spur Big Improvements in the SPD and Beyond
By David L. Taylor III, MSN, RN, CNOR
This column originally appeared in the April 2021 issue of Healthcare Hygiene magazine.
As the healthcare industry seeks answers to improve its “system of care,” an area often overlooked is sterile processing (SP). Far too often, administration fails to understand the importance of such a department, which supports and enables hospitals and health systems to focus on what they do best—delivering patient care. Without the SP function, a hospital can become paralyzed; therefore, it is vital that hospital administrators engage more readily with their SP professionals, so they can focus on improving processes and outcomes that impact the continuum of care.
One CEO did just that by engaging a team of consultants to improve both the operating room (OR) and SPD at two of his hospitals located in the Northeast—and the efforts were greatly rewarded. Initially, the SPD had been identified as a leading cause of the OR’s issues; however, after an intense multi-week assessment, it became clear that was not the case. While the SPD certainly had some issues that needed addressing, the OR team could not get out of its own way to realize they were part of the problem. More specifically, bullying behaviors from some in the OR were causing a divide between the two departments, which proved to be the root of the problems. Compounding matters further, after decades of neglect, understaffing, poor instrumentation maintenance and instrument set shortages, the SPD could not keep up with the demands placed upon them by the OR.
The fallout was significant. The SPD’s manager resigned, and the department had just failed its survey from the Joint Commission (TJC), and the SP team bore staggering responsibilities that included not just meeting the needs of the busy OR, but also all procedural areas (e.g., the Emergency Department, Intensive Care Unit, both onsite and offsite clinics, and an ambulatory surgery center). Additionally, the SPD was responsible for the cleaning, maintenance, storage and distribution of durable medical equipment, crash, trauma and hemorrhage carts, and it also was largely responsible for resupplying all nursing units and ancillary support areas with patient-related supplies through a modified materials management support system. The entire hospital relied on the SPD for nearly every aspect of patient care and yet the department had 10 full-time equivalent (FTE) vacancies, which greatly exacerbated the challenges of effectively and efficiently meeting organization-wide demands. The cause and affect due to the lack of staffing involved near-constant interruptions (occurring six times an hour, on average), staff burnout, errors and safety issues.
Through a collaborative effort with the hospital C-suite, a plan was implemented to address all issues identified during the assessment. First and foremost was addressing the TJC survey deficiencies that were levied against the SPD. To manage the improvement project, a new leadership team was needed. After an in-depth evaluation of the current staff, no one was identified who could step into a manager’s role. As a result, two interim managers and two supervisors were hired, one manager-supervisor set for each campus. Additionally, an interim SPD educator was employed to address educational needs at both campuses.
To address the survey deficiencies, an action plan was developed and each TJC issue was identified, prioritized and addressed. Areas identified included safety, cleanliness, clutter, employee skill level, and infection prevention issues. In conjunction, the leaders needed to evaluate and “know” their staff. Six-part folders were created for each employee, which allowed the interim leadership team to understand every employee’s employment history and better track their progress. Each folder contained:
• Hire date (work anniversary) and employee profile (job description, roles and responsibilities)
• A competency skills checklist and validation for each area in the SPD
• Mandatory training (BCLS, fire safety, etc.)
• Certifications held or in progress (e.g., CRCST, CIS, CER, CHL)
• Education and in-services, staff meetings, and continuing education credits; and
• Employee development (conference attendance, annual evaluations, counseling documents)
These efforts not only allowed the management team to create a feedback cycle (which demonstrated to each employee how they were contributing to their own department) but also allowed the SP team to more readily see how they and their department’s contributions impacted the organization. Put simply, it helped demonstrate their value to the organization—and showed that those in the C-suite also understood their importance. Further, the folders allowed the new management team to reconstruct every employee’s work history and have it at their fingertips. Some employees had worked in that facility’s SPD for more than four decades, yet no records were found in the department or with Human Resources (HR). The new folders allowed employees to take ownership in the process and they were asked to bring in copies of their certifications, continuing education participation, and more. As part of the ongoing assessment, training and mentorship, employees with strong skills were recognized and recommended for new service lead positions. In all, four of these new positions were created to accommodate day, evening, overnight and weekend shifts.
The educator began the process of validating the of skills of each employee, reeducating, as necessary. A new, on-the-job training program was developed to ensure current and new/incoming employees are trained to the same standard to meet current best practice standards. Competency checklists were created for each area of the SPD; all employees went through each task and their skills were evaluated. As a result, an action plan was created which helped generate ongoing education and in-service planning as well as the development of a formalized didactic training program that allowed employees to prepare for the certification exam.
Traveling technicians were also a part of the improvement plan. Because the SPDs at each campus had significant FTE vacancies, 10 traveling technicians were hired temporarily to fill in the gaps, each with their start dates staggered over several weeks. As a result, OR technicians who previously assisted with the workload in the SPD, despite being inexperienced in SP functions, were no longer needed.
To build a stronger bridge between the SPD and the OR, the interim management team began attending the OR’s morning huddle, which allowed the SPD team to hear firsthand any issues the OR was experiencing—and to be able to act on those issues in real time. Initially, the OR team seemed reluctant to have the SPD participate in their huddles, but they soon came to appreciate their presence. Over time, all SPD staff were brought to the OR to participate in the huddles. This helped them understand what occurred in the OR, while also allowing those in the OR to better know their partners in surgery from the SPD.
After each OR huddle, the SP professionals in attendance would round on every surgical suite to ensure each room had the necessary instrumentation to start their cases. When instrumentation was missing, the SP staff would search for those instruments, so the OR staff could continue opening their cases. This not only helped reduce frustration within the OR, but also helped those in the SPD uncover root causes to problems and more quickly work to remedy them.
The interdisciplinary huddles led to the following discoveries that were then addressed:
• Preference cards were out of date and did not represent the needs of the OR
• Lack of instrumentation for the number of same/similar cases scheduled for the day
• OR staff hoarding or hiding individual instruments and instrument sets because of mistrust
• Misuse of emergency carts, leaving them bare and not reporting their deficiencies to those in the SPD
• Using the majority of single-use peel packed instruments as user preference instead of adding necessary items to instrument sets
The need to find qualified SP candidates to permanently fill the vacancies was a top priority for the management team. Over several weeks, the leadership team interviewed candidates but were unsuccessful. To widen the pool for potential candidates, a plan was implemented to set up a job fair in a bordering state. The organization’s leadership knew it would need permanent, skilled SP professionals in order to make the process improvement changes sustainable. To entice the best candidates, they were offered paid relocation, a hiring bonus, a fair compensation package, and paid housing for 90 days after the agreed start date. Along with the compensation package, the hospital also agreed to provide a comprehensive training and education package and committed to pay for the employees’ certification.
The efforts paid off in spades. During the 12-hour job fair, 120 candidates were interviewed and 60 were offered a second interview. After the second round of interviews, 18 candidates were offered positions and 13 accepted. Upon completion of the employees’ orientation and training, 10 remained on the job and 100 percent became certified.
The six-month consulting project uncovered numerous issues and challenges that needed to be promptly addressed for the healthcare organization to continue meeting its patient care and service demands. This project also helped the organization’s administration see firsthand the hard work, skill sets, tenacity and vital contributions the SP professionals demonstrate each day.
It can be challenging to predict the wide range of situations a well-supported SPD will be able to support over time. Knowing the issues is the first step, but it is also important to understand that new issues will surface and need to be proactively addressed over time. It takes time to rectify sometimes decades of neglect; however, as this case study proves, a strong leadership and a team of skilled consultants to help steer the process can lead to significant improvements in a matter of months.
David L. Taylor III, MSN, RN, CNOR, is an independent hospital and ambulatory surgery center consultant and principal of Resolute Advisory Group LLC in San Antonio, Texas. He has been serving as an IAHCSMM author since 2019. He may be reached at david@resoluteadvisorygroup.com.
Advanced Certification Can Enhance Quality, Safety and Your Career
By Julie E. Williamson
This column originally appeared in the March 2021 issue of Healthcare Hygiene magazine.
The number of individuals who currently hold the Certified Registered Central Service Technician (CRCST) designation through the International Association of Healthcare Central Service Materiel Management (IAHCSMM) currently tops 34,800, and thousands more have chosen to advance beyond their CRCST by becoming a certified instrument specialist (CIS), certified healthcare leader (CHL) and/or certified endoscope reprocessor (CER). In fact, at the time of this writing, nearly 1,400 have attained three IAHCSMM certifications (known as Triple Crown) and nearly 500 more have earned all four IAHCSMM certifications (Golden Crown).
Many sterile processing (SP) professionals who have earned multiple certifications say they have seen their confidence, credibility and skill sets soar—and many also report that the additional certifications have helped them advance their professionalism and enjoy new career growth opportunities.
“One of the greatest gifts a sterile processing professional can give him or herself is a rewarding career,” says IAHCSMM education director Natalie Lind, CRCST, CHL, FCS. “Attaining additional certifications adds to their knowledge and skill set and in doing so, shifts the focus from having a job—with is a temporary mindset—to having a career, which is more of a lifelong philosophy.”
Attaining the foundational CRCST certification is a prerequisite before going on to earn the CIS and CHL designations; the CER, however, can be attained as a standalone certification. Whichever certifications an individual holds, many agree that the primary goal of attaining the professional distinction is to improve service excellence, safety and quality through broader knowledge and deeper understanding and skill development.
“Sometimes, I think we lose sight of the real goal of attaining certification,” notes Lind. “It’s a learning process designed to develop and enhance our skills and knowledge. Understanding the rationale for what we do makes us more confident and able to grow within the profession.”
Certification also helps ensure SP professionals stay current with best practices, standards and regulations—and for some, the decision to seek and attain advanced certifications is a deeply personal one, rooted in the desire to serve to one’s highest capability in the name of quality care and patient safety.
“I have challenged myself to be the best advocate for the patient I can. This is personal for me, not just a job,” says Jan Prudent, BA, CRCST, CIS, CHL, CFER, sterile processing manager at Eastern Idaho Regional Medical Center. She explained that to be truly successful in her role, she needed to learn the intricacies surrounding the services she and her team provided to their healthcare customers, and that meant also understanding the processes and reasons why the processes and steps were so important.
“[This] is essential to provide best practice to our patients and other customers. With that knowledge, I speak with authority, I am able to teach and coach others, and I am able to promote a profession that affects every facet of patient care,” she said. Prudent considers certification a measurement of success that serves as a baseline competency of knowledge and understanding that can grow and expand to drive improved technology and outcomes.
Planning ahead for one’s own career growth is always a wise move, and for SP professionals, certification can help them more easily reach the higher rungs on the career ladder.
“The time to prepare for a promotion is not when a position becomes available, but before, so when the opportunity presents itself, you are ready,” Lind said.
IAHCSMM past-president Tony Thurmond, CRCST, CIS, CHL, who serves as sterile processing manager for Dayton Children’s Hospital in Dayton, Ohio, first became a CST while serving in the U.S. Air Force; it was a role that also included serving as an SP technician. He wasn’t introduced to the CRCST until he was in the civilian sector, but he jumped at the chance to seek certification. “With IAHCSMM, I had the opportunity to gain multiple certifications to help elevate my career,” he said.
After attaining the CRCST, Thurmond found the opportunity to further advance his professional development was too good to pass up. He earned his CHL because he knew he wanted to move into a management role, and he was amazed how quickly he received unsolicited calls of interest from facilities across the country who were seeking SP managers.
In Prudent’s facility, a career ladder program was implemented for the department, with each certification serving as a “rung,” all the way through the supervisory role, which encompasses the CRCST, CIS, CHL and CER. The facility integrates the CRCST course work into new-hire shadowing and hands-on training.
For all the merits of certification, the most notable is the potential for improving customer service and patient outcomes. As Prudent points out, the mission for Eastern Idaho Regional Medical Center is to “improve the lives of those we touch.” It’s a mission she personally strives to perfect daily on the job.
“I cannot get there if I do not know how, and certifications are the stepping-stones to finding the way. You cannot fix what you do not know, and you cannot hold others accountable without first holding yourself to those standards.”
Julie E. Williamson is the director of communications/editor for IAHCSMM.
AAMI Updates ANSI/AAMI ST79; ASGE and Others Revise Multi-society Guideline for Flexible Endoscope Reprocessing
By Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS – IAHCSMM Clinical Educator
Editor's note: This column originally appeared in the February 2021 issue of Healthcare Hygiene magazine.
Last month, the Association for the Advancement of Medical Instrumentation (AAMI) released an updated version of standard ANSI/AAMI ST79 Comprehensive guide to steam sterilization and sterility assurance in health care facilities. The revised document includes the following four amendments:
Amendment 1: Environmental Services/Fans/Food and Drink
A new section was added in section 3.2.1.1 Design considerations that specifically states that food and drink should not be permitted in any area where medical devices are processed, as they can contaminate prepared and sterilized items, and attract insects.
This new section also requires that the sterile processing department (SPD) or other areas that perform sterile processing functions should follow the same housekeeping procedures as those used to clean operating rooms and delivery rooms. It is recommended that floors and horizontal work surfaces be cleaned at least daily. Other surfaces in an SPD, such as walls, storage shelves and air intake and return ducts, should be cleaned on a regularly scheduled basis and more often, if needed. Stained ceiling tiles should be replaced. High-level environmental cleaning should also be routinely performed and include ceiling vents, tops of equipment and workstation lights. Low-level environmental cleaning should occur on surfaces such as base boards, sterilizer base and cart bottoms. Lighting fixtures or covers should be cleaned at least once every six months.
It is recommended that a cleaning schedule be implemented and followed. A cleaning checklist may be used to demonstrate appropriate cleaning is being performed. A sample of an environmental checklist has also been added to the revised standard.
Cleaning verification tools should be used to measure the adequacy of environmental cleaning on work surfaces. These tools include ultraviolet visible markers and ATP bioluminescence. The metrics from the cleaning effectiveness should be provided as feedback to personnel performing the cleaning task as providing this measurement can improve the quality of cleaning.
Additions were also made to section 3.3.5.5 Heating, ventilation, and air conditioning (HVAC) operating parameters. The ventilation recommendation was changed to direct the healthcare facility to identify which version of ANSI/ASHRAE/ASHE 170 will be used, based on when the HVAC system was initially installed or last upgraded. The healthcare facility should establish and implement systematic processes for monitoring HVAC performance parameters and a mechanism for identifying and resolving variances within the rooms throughout the facility where SP functions occur.
This amendment includes additional recommendations regarding the ventilation systems, including those pertaining to the use of fans and the effects of doors and windows. The environment used to process and store medical devices has an impact on the safety of the devices processed; for that reason, it is important to have control of bioburden and environmental contaminants. The use of down-draft-type air circulation systems limits contamination by carrying contaminants toward the floor and away from work surfaces. Fans are not permitted in any sterile processing area because they create highly turbulent airflow, which recirculates dust and microorganisms from the floor and work surfaces—this, interfering with designed airflow characteristics. Windows and doors that affect the ventilation and airflow should be kept closed to further limit the risk for environment contamination.
Amendment 2: Inspection of Insulated Instruments
Two new sections have been added to Section 8 Preparation and assembly of instruments.
Section 8.2 addresses the inspection process to emphasize the fact that damaged instruments or incomplete instrument sets/trays may cause a delay or cancellation of a surgical procedure, and/or increase risk of patient harm related to instrument malfunction. Several steps can be taken to prevent such incidents. It is recommended that every time a medical device is processed, it should be visually inspected for cleanliness and integrity. Enhanced inspection with magnification, borescopes or other inspection methods to verify cleanliness and integrity may be used. A method should be in place to ensure the cleanliness and integrity of every instrument and medical device. Upon inspection, medical devices with retained soil or residue should be subjected to repeated cleaning and decontamination processes until the device is completely clean. Damaged instruments should be removed from service (damaged instruments should be addressed according to the healthcare organization’s policies and procedures). Methods used to change instruments from their original state—such as using an engraver that can cause fractures or surface damage—should not be performed. To ensure the correct type of inspection equipment is available, it is recommended to review the equipment instructions for use (IFU); this should also be done when purchasing new devices.
Section 8.2.1 addresses the inspection of instruments intended to be used with electric current (otherwise known as electrosurgical instrumentation). Electrosurgical instruments are insulated and, typically, used in minimally invasive surgery. They are susceptible to physical and mechanical damage and degradation related to repeated use. Using insulated instrumentation with holes in the insulation or other defects places the patient at risk for significant harm. The holes may not be clearly visible; if they are not detected during instrument processing and the damaged instruments are used during a procedure, the insulation breach can result in an electrical current escape that can burn the patient.
Insulated instruments should be carefully inspected, including their cords, which can become damaged during normal use, processing, contact with sharp instruments, and use of high voltage electricity. It is recommended to organize instruments (such as in instrument sets) to protect them from damage.
To prevent patient harm, insulation testing for insulation integrity is recommended each time the instruments are processed, in accordance with the instrument manufacturer’s written IFU for inspection. There are different types of insulation testing methods and they vary with insulation tester type. There are also a variety of accessories to test specific instrumentation and cables/cords, based on their design. For that reason, it is vital to refer to the instrument and insulation tester manufacturers’ written IFU for their recommended procedures. Insulation testers are designed to detect small current leaks that can jeopardize patient safety. Any time instrument insulation does not pass inspection, the device should be immediately removed from service, and damage should be addressed according to the healthcare organization’s policies and procedures that address instrument evaluation, repair or replacement.
A table was added that addresses the inspection points and possible damage for various instruments/devices. This table lists the name of the instrument/device, followed by its inspection points, as well as the possible damage to the medical device, and methods to assist with inspection/testing. This quick reference inspection guide will be helpful as these instruments are processed.
Images of instrument and cord failures are also provided to help present the reader with a clearer understanding of what to look for in electrosurgical instrumentation and cord failure.
Education is the cornerstone of good practices. That fundamental understanding is reflected in this amendment, which recommends that personnel responsible for processing these instruments should receive education to ensure they understand how to use testing equipment safely and effectively. In addition, competency should be verified and documented before employees’ first assignment to use the equipment.
Amendment 3: Modification of Content Pertaining to Frequency of Cleaning for Routine Care of Sterilizers for Sterile Processing Areas in Health Care Facilities
This amendment changes the recommendations in section 12.4 Routine care of the sterilizers. The previous version recommended daily inspection and cleaning. The revised recommendation directs the user to follow the sterilizer’s IFU, which may require that weekly or other prescribed inspection and cleaning be performed (and it should be documented, per internal procedures).
Amendment 4: Content Addressing Recording Biological Indicator Lot Numbers in Sterilizer Records for Sterile Processing in Health Care Facilities
This amendment revises the wording in sections 13.8.2.3 BI PCD test procedure, 13.8.3.3 Test procedure and 13.8.4.3 Test procedure. The previous wording of these sections did not specifically state to record the lot number, only the “number of both test biological indicators (BIs) and control BIs.” To better clarify the specific “number” to document, the revised wording specifically states to document the lot number of both test BIs and control BIs.
The amended standard is available at www.aami.org/ST79. ANSI/AAMI ST79:2017 Comprehensive guide to steam sterilization and sterility assurance in health care facilities will be automatically updated for users with an AAMI eSubscription. Those who previously purchased the printed version or have a PDF version will receive a PDF of the amendments via email at no cost.
Multi-society Guideline for Flexible Endoscope Reprocessing Updated
The American Society for Gastrointestinal Endoscopy (ASGE), along with members of the American Society for Gastrointestinal Endoscopy Quality Assurance in Endoscopy and Standards of Practice committees have updated the Multisociety guideline on reprocessing flexible GI endoscopes and accessories. This guideline was revised using evidence-based recommendations, based on rigorous review and synthesis of the present-day literature—using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. The GRADE framework is an all-inclusive and transparent system for the quality rating for the evidence and strength of the recommendations. What follows are highlights of this revised guideline.
This guideline states that staff training and competency of endoscope reprocessing skills are important aspects of infection prevention. It is recommended that reprocessing staff be trained on all the endoscope models they are expected to process and shown to have documented competency before being assigned to process them. The healthcare organization should then perform competency evaluations of endoscope processing personnel on a scheduled basis, as defined by the organization (typically, this occurs when one first assumes endoscope processing duties; at least annually; anytime a breach is identified; when a major technique or new endoscope or reprocessing accessory is introduced; and in the context of local quality control efforts).
The delayed processing of endoscopes has become a hot topic. This guideline recommends that manual cleaning begin according to the instructions for use (IFU), which is usually within an hour after the endoscope is released from the procedure. If cleaning is delayed beyond this time period, the manufacturer’s IFU for delayed processing must be followed. It is recommended that soiled endoscopes be transported immediately to the reprocessing area in a fully enclosed, puncture-resistant, leak-proof containers with a biohazard label.
It is recommended that only clean cleaning implements are used; if reusable cleaning implements are used, they must be cleaned and disinfected between uses. The guideline also recommends the use of fresh cleaning solution for each endoscope. If the cleaning solution falls outside the recommended temperature and dilution range, it is recommended that it be replaced.
If an endoscope undergoes high-level disinfection, it is recommended to perform this disinfection step in an automated endoscope reprocessor (AER) using a high-level disinfectant or sterilant that is compatible with the AER and sterilizer IFU. The importance of adhering to the endoscope manufacturer’s IFU was emphasized. Some duodenoscopes contain an elevator wire channel that may not effectively be disinfected by some AERs and that this step should be performed manually. In addition, the endoscope and components should be attached using only approved connectors, per the AER and endoscope manufacturer’s IFU to ensure contact of all internal surfaces with the high-level disinfectant solution. If the AER cycle is interrupted, the entire cycle should be repeated.
Recent research links incomplete endoscope drying to multiple outbreaks of waterborne organisms (data shows a reported endoscope contamination rate of 80 percent). This research signifies that the methods of drying flexible endoscopes need to change—and this multi-society guideline includes new drying recommendations. Prior to this newfound information, 70 percent to 90 percent ethyl or isopropyl alcohol was injected into the endoscope channels to dry the channels. Alcohol was recommended because it purged and promoted the evaporation of residual water within endoscope channels, thereby decreasing the chances for bioburden buildup. There is little data on the possible benefits of alcohol flushes. Based on research, this guideline recommends drying the endoscope channels and areas not dried with a cloth with forced, pressure-regulated filtered air— with a sufficiently prolonged flow of medical air through all accessible channels. For best results, it is ideal for this step to occur simultaneously for all channels and for a duration of at least 10 minutes. Flexible endoscopes should be completely dried after processing and before use. Note: This guideline recommends following manufacturers’ IFU regarding the use of ethyl or isopropyl alcohol for drying endoscopes. There was a case report that switched to suctioning 70 percent alcohol through a duodenoscope working channel, followed by compressed air during the drying phase. It was reported that this helped contain an outbreak of Pseudomonas aeruginosa. In addition, a conclusion from the pulmonary literature suggests that using alcohol for drying purposes significantly reduces bronchoscope contamination rates; however, the fixation properties of alcohol could lead to the retention of organisms within the endoscope. At this time, there is no data to strongly support or refute the use of alcohol flushes for the drying of endoscopes.
Proper storing of endoscopes is important to prevent contamination. This updated guideline includes information on proper endoscope handling, including the need for personnel to perform hand hygiene and wear clean gloves during all phases of endoscope handling. Endoscopes may be stored in drying cabinets or conventional cabinets, and always in a way that does not allow for moisture to collect on or within the endoscope.
Drying cabinets have connectors that force air through each endoscope channel. The endoscopes can be stored either vertically or horizontally. These storage cabinets use forced irrigation of endoscope channels with warm, filtered air during storage to achieve complete drying of the channels. This reduces the proliferation of Pseudomonas aeruginosa; however, these cabinets’ importance for keeping endoscopes free of contamination remains incompletely defined.
Conventional cabinets require endoscopes to hang vertically, and active or passive ventilation with filtered air helps prevent moisture from forming on or within endoscopes. Passive cabinets without airflow directed into all channels are not sufficient for drying the endoscope from a wet state.
Endoscope cabinets should be in a secure location that is near but not in the procedure rooms. Care and maintenance of the cabinet should be performed according to the cabinet IFU and the cabinets should be routinely inspected for damage and cleaned on a routine basis (and when soiled) with an Environmental Protection Agency–registered hospital disinfectant. This guideline does not provide a specific amount of time that endoscopes can be stored before being considered contaminated. It is recommended that Endoscopy units evaluate the available literature, perform an assessment regarding benefits and risks around the optimal storage time for endoscopes, and then develop a policy and procedure specific to their unit on endoscope storage time.
The multi-society guideline also addresses recommendations regarding the use of simethicone. It is recommended that the Endoscopy unit follow the manufacturer’s IFU on its addition in water bottles and irrigation devices, including cleaning and disinfection of endoscopes after its use.
The guideline also recommends that documentation be put into place to provide traceability of endoscopes, including those that are loaned.
This article has provided some highlights of the updated Multisociety guideline on reprocessing flexible GI endoscopes and accessories. The complete guideline is available on the ASGE website at www.asge.org (under the “ASGE guidelines” and the “Newly Published” tab).
Susan Klacik, BS, CRCST, CIS, CHL, ACE, FCS, serves as a clinical educator for IAHCSMM.
Poorly Managed Loaned Instruments Pose Potential Infection Risks
By Julie E. Williamson
This column originally appeared in the January 2021 issue of Healthcare Hygiene magazine.
Proper management of loaned instruments and trays ranks among the top challenges for many sterile processing (SP) professionals – and it’s an obstacle that’s only become increasingly difficult to hurdle in light of rising procedural volume (e.g., for orthopedic, spinal and endoscopy cases, to name a few) that commonly up the odds for more loaned instruments making their way into the SP department (SPD).
Loaned devices are often more complicated and sophisticated, and processing them properly and safely hinges on the availability of proper resources that include adequate time for advance staff training, proper types and amounts of equipment and supplies, ample storage space, ready access to the most devices’ current instructions for use (IFU), and the need for loaned instruments to be delivered far enough in advance of the procedure to ensure thorough processing can be performed. Unfortunately, far too many healthcare organizations and vendors aren’t doing their due diligence to ensure all those critical needs are consistently met. It’s a shortcoming that can jeopardize patient safety, increase infection risks and cost the facility dearly on numerous fronts.
“There are no easy answers or silver bullets, but having an understanding of why things happen the way they do in the loaned instrument world is critical, along with the understanding of how we as professionals can make it better, not only for the surgeons and patients but also for our staff,” said Damien Berg, BA, BS, CRCST, AAMIF, regional manager of sterile processing for University of Colorado Health, during his IAHCSMM Online Conference session, “Loaned Instruments: Proper Protocols and Challenges.”
Berg, an IAHCSMM past-president (2018-19) and voting member and co-chair for documents from the Association for the Advancement of Medical Instrumentation (AAMI), has spent more than 27 years working in the operating room (OR) and sterile processing department (SPD). Today, he oversees two large UCHealth medical centers and a surgery center, both of which regularly receive a steady stream of loaned instruments. In his many years of experience, he’s faced (and improved upon) numerous challenges related to loaned instruments and he’s now frequently asked by his SP peers to share his advice on how best to manage those devices.
What follows are some of his top tips:
1. Establish a detailed policy for managing loaned instruments throughout every step of the cycle – and ensure all stakeholders (SPD, operating room, purchasing, infection prevention, and vendors/distributors) comply and are held accountable. Numerous resources are readily available to help guide the policy development process, including AAMI Technical Information Report (TIR) 63, Management of loaned critical and semi-critical devices that require sterilization or high-level disinfection, and free sample policies and procedures on loaned instrumentation available from IAHCSMM (https://www.iahcsmm.org/resource-documents/loaner-instrument-template.html).
“These documents can help us with managing the process and ensuring we are covering all the critical pieces, as well as helping ensure we’re treating loaned instruments as well as would with our own instruments,” Berg explained, adding that it’s prudent that all stakeholders receive a copy of the facility’s/organization’s policy and sign off stating that they read the policy and agree to the outlined terms.
2. Have dedicated SP team members on appropriate shifts who are trained on the loaned instrument policy and how to properly manage receipt/release of loaned sets. It’s also important that all stakeholders understand that all loaned instruments that arrive to the facility will be treated as contaminated and always undergo full reprocessing, in accordance with IFU and industry standards. No instruments will ever arrive and head directly to the OR or other user area, regardless of how they were handled or transported prior to arrival.
3. Never rush the process. Time requirements and drop-off expectations for loaned instrument must be clearly outlined in the policy and then stressed to all parties to ensure compliance. While SP professionals are typically not part of the stage where loaned instruments are requested, they must be involved in the arrangement as soon as possible to ensure the SPD has the proper equipment, supplies, instructions for use, etc. to ensure they can safely and effectively process the items prior to the procedure (and within the timeframe stipulated in the policy).
During his session, Berg explained it this way: “If Dr. Smith is doing a total knee, for example, Company X will be bringing in a certain number of trays for the procedure, and we know that if the instruments are needed in the OR by Tuesday at 7 a.m., we’ll need those total knee trays received in the SPD by Monday at 7 a.m. because our facility’s policy states instruments must arrive at least 24 hours before the procedure.” He went on to explain that if a drop-off occurs last-minute or at any point under 24 hours, the time of actual delivery (as well as the reason for the late arrival) should be documented. Such documentation helps identify trends and, perhaps, gaps in communications or compliance, which can be more proactively addressed – and surveyors will also be looking to ensure receipt of loaned instruments is documented and comparing that to the facility’s written policy. “If you have a 24-hour or 48-hour policy on when loaned instruments need to arrive to your facility [in advance of the procedure], but surveyors discover that’s not being followed, you’ll be in violation of your own policy.”
4. Don’t let loaned sets tip the scales. Loaned instrument policies should also clearly address weight limits, which are reflected in the AAMI standards and Association of periOperative Registered Nurses (AORN) guidelines. Both recommend that instrument sets and trays prepared for sterilization not exceed 25 pounds, and that weight includes the container that holds the instruments. Berg recommends SP professionals not only state in the policy that 25-pound limit, but also working with vendors to determine how heavy sets can be brought down to a more manageable weight, perhaps by splitting the contents into two trays or working with the OR to determine if any instruments in the set that typically go unused can be removed from the tray and packaged separately.
5. No IFU or vendor education? No instrument acceptance. Without ready access to current instructions for use (IFU), SP professionals simply cannot properly and safely manage and process instruments. IFU and additional training requirements (as needed) should be directly stated in the loaned instrument policy, and they should then be reiterated with vendors. Ahead of all procedures where loaned devices are to be used, the SP team just also verify they have the proper equipment, supplies, IFU and other essential resources needed to reprocess those instruments safely and thoroughly. Not only should technicians understand the cycles needed to reprocess the loaned instruments, they must also have knowledge of how the devices are disassembled to facilitate proper cleaning.
“IFU need to be provided for all instruments, no exceptions,” Berg says. Once those instructions are provided by the vendor, copies must be made for SP professionals across all areas of the department, from decontamination and assembly to sterilization and storage.
6. Inventory carefully – and take photos as proof. At any point through the the use, handling or delivery cycle, loaned instruments can become lost or damaged, and that can prove costly for the user facility if diligent documentation isn’t happening at the point of instrument receipt and release. Berg said ensuring a manual or electronic count sheet is readily available at the location where loaned instruments arrive and leave the facility is crucial for tracking all set contents, and he stressed that list/count sheet should never be signed/approved without visual confirmation that all contents are not only in place and not damaged. Photographing set contents is a simple step that can pay big dividends.
“Pictures are worth a thousand words,” said Berg. “If an instrument turns up missing or obviously damaged after it’s left your facility, imagine the benefit of being able to visually show they were present and undamaged at the time of pick-up.”
Julie E. Williamson is the director of communications/editor for IAHCSMM.
Measure 2020’s Triumphs and Pitfalls, Chart Course for Progress in 2021
By Nicholas Schmitz, PMP, LSSBB
This column originally appeared in the December 2020 issue of Healthcare Hygiene magazine.
At the end of each year, I always recommend using it as a time to closely reflect on what transpired in the department over those 12 months (doing so may even be a requirement from your healthcare facility, particularly the need to review organizational metrics, which makes a more comprehensive assessment doubly important). This type of review is valuable because it not only shows us where we and our processes/practices might have fallen short, but also reminds us of what worked well that could be carried into the new year.
The following key questions can help make the most of this year-end review and, ideally, help guide the process in a more organized and thought-provoking way. You’ll also find some best practices that can help you avoid pitfalls in this assessment journey.
What went well? Review your last 12 months and think about all of the things that went well. It is natural and all too easy to focus on the negatives, especially in a year like 2020 when the hits just seemed to keep coming. But if we really take the time to examine what transpired, we’ll see some strong positives rise to the surface. Beginning with some positive reinforcement will also make addressing some of the negatives a bit easier.
Who needs to be acknowledged for jobs well done? Sometimes, we get so bogged down in the processes and outcomes that we forget to thank those around us who helped drive success. Think about the things that went well and the people or played a part in that, and then be sure to let them know they’re appreciated. At the same time, think about things that may have initially gone wrong but were set on the right path by bright ideas and well-delivered contributions of members of the team.
What didn’t go very well? Identifying the year’s pain points can be less than fun, but examining failures is time well spent (and choosing to skip over this step can lead to stagnation and further failures, which can impact service quality and efficiency and, more importantly, patient care). It’s important to realize that humans tend to learn more from failure and difficulty than they do from success and ease. The best way to approach this step/question is by tackling it as objectively as possible. It’s not even necessary to assign a cause or blame to that year’s failures; merely acknowledging something didn’t work well can be a powerful enough first step to help lead to correction.
With the book closing on 2020, we can – and should – set our sights on the new year (which many of us will gladly welcome). Having a list of what worked (and is continuing to work) well is an excellent place to start when considering departmental goals for the new year. While there will always be core tenants to the job, such as sterility and function, other areas of focus may come and go, as needed. The goal setting process used at General Electric (GE) – called SMART – is one that can be applied to healthcare as well. SMART stands for: Specific, Measurable, Achievable, Realistic, and Timeline.
Breaking sterile processing-related goals into these SMART components can be the difference between hoping something happens and finding a way to make it happen. Still, simply focusing on these goals may be insufficient because it is possible to follow the process without focusing on critical areas of success. Because of this, GE added stretch goals to identify areas that are both challenging and important. The key to a stretch goal is for it to be difficult but not so far out of reach that it crushes morale. Here are some common pitfalls to be aware of and try to avoid:
Be careful what you measure. Choosing metrics to associate with objectives for the year requires thoughtful consideration. A good story that highlights how goal setting can go awry comes from the world of sports. Vasili Alexeyev, a Russian super-heavyweight weightlifter, was offered an incentive for every world record he broke. The result was that he kept breaking world records (often, only his own records) by a gram or two at a time to maximize his reward payout. In this example, it becomes easy to imagine how measurement in one area can drive poor results in another.
Don’t measure the wrong things. Dysfunctional measurement is often caused by organizations doing the wrong things for the right reasons. Consider this example: In order to reduce customer’s wait time, an insurance company known for its customer focus invested in a device to measure average customer wait time for each call center team. They mounted a digital scoreboard above the office cubicles for all to see, which caused employees to get their customers off the phone quickly, even if their issues had not been completely resolved, just so that the customers in the queue wouldn't have to wait. It even compromised customer service behaviors such as empathy for a customer who had recently experienced a death in the family. Fortunately, when the CEO realized the problem, the "wait time" measure was immediately replaced with one that measured the percentage of customers who completed their business on the first call, with no need for follow-up.
Before finalizing goals and metrics, discuss them thoroughly. The process of goal setting has a powerful effect on motivation; therefore, I encourage all leadership to involve their team, peers, and customers in the process. Consider this example of measurement gone wrong: An automobile industry executive explained that to receive his quarterly bonuses “all that mattered was meeting production quotas and getting the cars out of the factory.” What happened after that was somebody else’s problem (certainly not a good way to work). Imagine the harmful results if a similar approach was implemented between Sterile Processing and the Operating Room (OR). Ask your team which objectives and metrics could be set that would support OR goals and vice versa. How do those objectives align with the overall organizational goals? Do these align with the vision and values of the organization?
At the end of each year, it’s important to take an honest look how the team, department and organization performed – good or bad – and then use those assessments to help guide future goals and strategies for the months and year ahead. Continuing to examine and question the processes and metrics of the department will help ensure past failures don’t continue and necessary changes are set in motion.
Nicholas Schmitz, PMP, LSSBB, is president of Schmitz Consulting LLC and has served as an IAHCSMM contributing columnist since 2015. He holds two master’s degrees in organization development and change management, and project management, and is a certified project management professional and Lean Six Sigma black belt.
Healthcare and Sterile Processing Professionals Can Lead Charge for Positive Change
By Norman Thompson, MBA, CRCST, CER, CHL
Editor's note: This column originally appeared in the November 2020 issue of Healthcare Hygiene magazine.
The first confirmed case of COVID-19 in America was January 20, 2020, and many of us still vividly recall the news broadcasts that informed us that this life-changing virus had landed in our homeland. Although it wasn’t deemed a pandemic at the time, that would change just weeks later – and along with that, our personal and professional lives would change along with it, in some notable ways. The pandemic forced the world to reevaluate how we interact in our daily lives, both in and out of the healthcare facility, and it’s also forced us to lessen the grip of “doing things as we always have.”
No question, the shifts perpetuated by the pandemic have caused chaos and unsettling concerns about not only what our day-to-day lives look like now, but what shape that will take moving forward. Although many questions remain to be answered, one thing we know is now is a time for unity and turning obstacles into opportunities for positive change -- and the healthcare industry can lead the crusade.
Teamwork will continue making our mission statements work, and achieving it in a healthy, ongoing way needs to be a top priority. Our critical need to work together, united in our common goal of patient safety and quality patient care, must stay at the forefront – not just today and tomorrow, but in the long-term, indefinite future. True teamwork is a pursuit that’s long been touted, encouraged and promoted, but it is not something that is always successfully attained. We might enjoy it during certain projects and for brief periods following training events and collaboration initiatives, for example, but for many facilities, it’s fleeting. The time is now to finally ingrain it into the fabric and lifeblood of our organizations, departments and individual selves.
When healthcare organizations’ rallying cry becomes, ‘We’re all in this together and are operating as a cohesive unit, not as separate professionals on different teams,’ our service delivery improves and positive outcomes result. Communication and commitment to education and professional development will help drive this unification and make our healthcare mission statements a mission, not just a statement.
SPD’s critical role
In many cases, the sterile processing department (SPD) still suffers from an identity crisis with other departments it serves; many simply are unaware of the work that takes place in the SPD, and the level of expertise needed to get the job done safely, efficiently and correctly. If a positive has come from the pandemic, it’s that it’s created an opportunity to reintroduce the services SP professionals provide and, in some cases, show how they can serve other departments in new ways.
It’s up to SP leaders to demonstrate their contributions and value through process and practice analysis and data capture/dissemination. The department should consistently implement and utilize proven analyses to build a robust environment and ensure that the team is working most effectively and efficiently. The two analytical methods I’ve found to be most effective in an ever-changing environment like the SPD are continuous quality improvement (CQI) and root cause analysis (RCA).
CQI is a defined quality management process that focuses on can increase departmental functionality, enhance employee and leadership development, and promote interdisciplinary quality. This process encourages all healthcare team members to continuously ask the questions, ‘How are we doing?’ and ‘Can we do better?’ and addressing these questions effectively requires structured clinical and administrative data.1 RCA provides insight into troublesome issues that cause delays in daily operations that ultimately affect service delivery and patient care. This analytical tool, if properly implemented, can identify solutions and strengthen accountability. Both of these process management tools can increase productivity and profitability. Even so, in the SPD realm, specifically, it’s important to recognize that some key foundational changes must occur before positive, long-term improvements can occur.
Heightened focus on education, certification
I believe that positive change within the SPD begin with understanding the essential roles of SP professionals. Those outside the department – from the operating room and other “user” departments” to infection prevention, risk management, human resources and those in the C-suite (among others) -- need to be educated about the SPD’s critical role in the delivery of quality patient care. These people should be invited to the department to meet the SPD team and see for themselves all that goes into instrument reprocessing. They must also understand the importance of developing a department of professionals that is rooted in ongoing education and certification, and ensuring SP technicians have the tools and resources they need to succeed on behalf of the department and all the others it serves.
I strongly advocate requiring SP technicians and those in leadership positions within the department to become certified in their roles. Recruiting certified technicians and compensating for advanced skills and years of service can help set the framework for success in the department and in all areas of customer service. These qualified technicians can become part of a leadership development pool, which further reinforces value in the healthcare facility. Certification helps ensure that SP professionals possess the essential knowledge and skills necessary for managing their many essential departmental duties safely, effectively and consistently. Additionally, the ongoing education required for SP professionals to maintain their certification status helps ensure that these professionals stay on top of ever-evolving instrumentation, technology, standards and best practices to keep SPDs functioning at peak performance.
This knowledge helps drive quality and practice consistency – both of which are positive outcomes that will help others within the facility see SP professionals as the vital teammates they are. It will also help those at administrative levels of the organization to better understand that the SPD is the engine that drives the OR and other critical patient care areas of the facility. Often, that knowledge and understanding will help facilitate better communication and improved resolution when challenges or problems arise.
Healthcare organizations need to recognize that, often, the most qualified people to lead the department are those skilled technicians who understand the processes, challenges and innerworkings. Organizations just need invest in these professionals’ leadership development and elevate them through mentorship, continuing education and advanced certifications.
Conclusion
The pandemic has brought more than challenges; it’s also provided an opportunity for change. This metamorphosis will continue to affect every aspect of operations within the healthcare segment. It’s up to each of us to embrace this change and turn it into a positive. As an SP technician with three certifications – CRCST, CER and CHL – I, personally, view this change as exciting and inviting. But we need more to lead this charge toward teamwork, recognition, professional development and the pursuit of excellence. Sterile Processing is a vital part of patient care and we must, as a team, be the leaders of this transformation.
Norman Thompson, MBA, CRCST, CER, CHL, is a sterile processing professional who has authored articles for the International Association of Healthcare Central Service Materiel Management since 2018.
References:
1. Health Information Technology Research Center. Continuous Quality Improvement (CQI) Strategies to Optimize Your Practice. National Learning Consortium. April 12, 2013.
Common SPD and OR Missteps Place Patients at High Risk
By David L. Taylor III, MSN, RN, CNOR
Editor's note: This column originally appeared in the October 2020 issue of Healthcare Hygiene magazine.
Surgical instruments, endoscopes and durable medical equipment that are of subpar quality place patients at risk every day. As a consultant, I have the good fortune of working with health systems across the country and I see a lot of good things and not-so-good things in this role. This article will share some of my experiences when consulting with numerous facilities, and will, hopefully, lead readers to examine their own practices and make necessary changes to improve quality and keep patient safety the top priority.
Today, hospitals are larger and more complex, with many moving parts. Everything works in tandem, until corners are cut (e.g., staffing, training, education) and those shortcomings lead to negative outcomes. For decades, thousands of patients have been negatively impacted by healthcare systems that neglected to properly support and manage the Sterile Processing department (SPD). Many healthcare systems don’t believe it will ever happen to them, but they are mistaken. We continue to see too many infections, retained foreign objects, life threatening injuries, and even death – all the result of actions that could have been avoided.
Healthcare leaders and their staff members who perceive their departments are running well are often surprised when a consultant comes in with a fresh set of eyes and tells them differently. It is not uncommon for organizations to be unaware of their problems because the jobs they perform are difficult, even under the best of circumstances. What follows are some of the most valuable lessons I routinely share in my consultant role:
Cleaning should begin at point of use. Bioburden such as blood and tissue, as well as medication and saline, are the primary causes of pitting, staining and discoloration of instruments. Instrument cleaning should begin during the surgical procedure to prevent blood, soil and debris from drying on the surface and within lumens. Point-of-use care means where the instruments are used. Operating Rooms (OR) have many time pressures and it is very easy to neglect cleaning and proper care of instrumentation used during a procedure. Blood and tissue that are allowed to sit and dry on an instrument make that device more difficult to process. Its presence can also cause pitting and other problems, reducing the instrument’s life expectancy and usefulness and costing hospitals thousands of dollars every year in expensive repairs and premature replacement. If bioburden isn’t properly removed prior to sterilization and that instrument is subsequently used on a patient, life-threatening infections can result. Remember, an instrument cannot be effectively sterilized if it hasn’t first been thoroughly and properly cleaned.
Tape is often misused. Healthcare workers do love their tape. Whether that tape is scotch, masking or medical varieties, it seems it is often used on everything except for which it was intended. Autoclave tape is used to secure packaging materials (e.g., wrapped, container or pouch systems), allow penetration of the sterilizing agent and maintain sterility of the processed item after sterilization. Autoclave tape consists of colored Kraft paper with a rubber resin adhesive that resists moisture and most solvents. The tape can withstand broad temperature and environmental extremes (including steam sterilization) and is not intended for internal use (it’s not validated for such use). The importance of this cannot be overemphasized because tape can block the disinfectant or sterilizing agent from making complete contact with the surface of instruments. Still, it is often being used to secure integrators and pouches on instrument baskets and trays. Load stickers and office-based label makers used to identify instrument sets are also being used inappropriately – and these practices are unacceptable. If tape is found on the inside of wrapped, container or pouch systems, it should be considered contaminated.
Rigid containers, blue wrapper and peel packs are often mishandled. Wrappers, containers and pouch systems have been used in our industry for decades. Unfortunately, many leaders and staff members do not understand the importance of their proper handling and care. They fail to recognize that containers, wrap and peel packs require as much care and attention as the instruments themselves.
In my consulting, I often find poorly maintained rigid containers in daily use that are dented and misshapen, with rubber seals either dry rotted or missing. Some containers I’ve found were in such bad shape, a sheet of paper could easily be passed between the container and the lid once it was “secured.” I have even found insects that made their way into rigid containers, along with dust and lint the color of the organizations’ scrubs. There was no telling how long these instrument containers had been compromised or how many patients might have been impacted.
Blue wrapper and peel packs are commonly used (and misused) as well. The presence of holes in these items is not uncommon and it compromises the integrity of what the products were intended to protect. Instrument sets, basins and other items packaged with blue wrapper and peel packs are often stored inappropriately, in high traffic areas, and often next to water sources or on shelves where employees rub against them as they pass by. I have even seen employees use the same shelves where instruments are being stored on as step stools to reach items located on higher shelves. The integrity of peel packs can be compromised when stuffed beyond capacity into drawers or bins. Peel packs can be packaged either with a single or double pouch; however, inner pouches must remain flat to ensure steam penetration is not compromised as a result of air pockets. This standard is more than a decade old, but I find peel packs all over the country that have inner pouches folded over, often not only once but several times. When package integrity is compromised, it is often blamed on the SPD. In reality, it is the responsibility of both the OR and SP professionals to properly handle and store these items.
SPD equipment and chemicals are often overlooked. Equipment used in the SPD must be inspected, monitored, tested and cleaned regularly. Chemicals used in the cleaning and disinfection process must be monitored as well. Unfortunately, this isn’t always happening. On a recent visit to an organization that was having issues with high surgical site infection rates, it was discovered they were not maintaining their equipment or using chemicals properly. Autoclaves were filthy, with years of dust built up behind the doors of the units and on the controls. Drain screens were also caked with hard water deposits, preventing the evacuation of air from the autoclave chambers. Efficacy testing (e.g., dart tests, biologicals, etc.) were not performed routinely or being documented. Chemicals (e.g., enzymatic and detergents products) were not routinely inspected. In some cases, containers were found empty and had been for weeks, which prevented proper decontamination to remove soil and organic matter – ultimately, placing both SPD staff and patients at risk.
Manufacturing of “homemade” medical supplies is occurring. During a consultation with one healthcare organization, I was horrified to discover “endoscopic cholecystectomy pouches” being dangerously made from Walmart sandwich bags (employees were placing them in peel pouches and sterilizing them to save money). This is wrong on so many levels. Did they not know they are required to attain 510(k) clearance from the US Food and Drug Administration (FDA) in order to manufacture medical supplies? Did they not realize there was no way to properly sterilize these sandwich bags because they were not designed for such a purpose (least of all, for use in patient care)? What made this worse was the decision to make these pouches from sandwich bags was initiated by the surgeon and sanctioned by the OR director who purchased the sandwich bags and instructed the registered nurses to create them (and all of this was done with approval from the chief medical officer and hospital president).
SPD staffing is often lacking. If hospitals advertised they have been understaffing and underfunding their SPDs for years and, as a result, were putting patients at risk for a life-threatening illness, they would not be in business very long. Still, many healthcare facilities are doing just that, and they’re jeopardizing the safety of their patients and employees and putting the organization’s reputation on the line in the process. Increasingly, headlines are being made when SPDs are neglected; budgets fail to allow for adequate equipment, training, staffing and other crucial resources; and mistakes and shortcuts happen as a result. When device contamination leads to patient injury, that’s considered a “never event,” meaning it should not have occurred. These events have been reported on The TODAY Show and across all major television networks, as well as in some of the nation’s most prominent newspapers, and those preventable incidents are costing hospitals millions of dollars. Healthcare leaders must proactively manage the day-to-day operations of the SPD if they wish not to become fodder for the next news story.
What is accepted is what is taught (good or bad). Standards of care and accountability matter. What employees see every day becomes the standard, so if what they see is wrong and their leaders do not correct that perception, those incorrect processes will surely continue. I’ve seen employees doing the wrong things simply because that was the way they were taught or “the way things have always been done.” When educating, it’s vital to train and validate employees’ competencies and to give them the tools to challenge the status quo. Industry standards, guidelines, IFU and policies and procedures must be followed diligently and consistently. Right from wrong must never be left to interpretation.
Conclusion
Patients are counting on everyone involved in their care, including those in the SPD, to do the very best job possible. It's time for facilities to elevate the standard of care being practiced in their SPDs and commit to making their facility the best choice in healthcare. For those who are unsure where to begin, consultants can help guide the process by using their breadth of knowledge and expertise to shed light on shortcomings and truly serve as a partner in quality. Unfortunately, many healthcare organizations only seek help when they are in trouble and by that time, it’s often too late for patients.
David L. Taylor III, MSN, RN, CNOR, is the principal of Resolute Advisory Group, LLC, a healthcare consulting firm in San Antonio. He has served as an IAHCSMM columnist since 2019. For more information email David@ResoluteAdvisoryGroup.com
Reference:
1. Taylor DL. Perioperative leadership: managing change with insights, priorities, and tools. AORN J. 2014;100(1):8-26, 27-29.
Difficult Times Deliver Opportunities for Lasting Positive Change
By Nicholas Schmitz, PMP, LSSBB
This column originally appeared in the September 2020 issue of Healthcare Hygiene magazine.
We are all facing some unprecedented times and unforeseen challenges this year, including those working in sterile processing (SP). Many of these professionals and their departments are functioning in unexpected circumstances and stepping up to do everything they can to help their healthcare customers, facilities and patients. Put simply, excelling during this trying time of adversity is another service they’re offering.
These difficult times are also challenging assumptions and habits. That can feel disruptive at first, but it can lead to positive outcomes when viewed and explored in a positive light and as an opportunity for growth and improvement. Now is an excellent time to get the ball rolling on changes the team/department has been wanting but, perhaps, hadn’t had an opportunity to tackle previously.
It’s clear that healthcare isn’t the only business segment facing strife and uncertainty. Businesses throughout the world have found themselves challenged to serve their customers within the context of our new reality. And they’ve had to reinvent and innovate to stay relevant and effective in the eyes of their customers and communities. Imagine being an establishment that can no longer allow customers to come inside. How these companies/businesses react and adapt will likely determine whether they survive in the days, weeks and months ahead.
One leading corporation has provided a blueprint other organizations and businesses can follow. In a national webcast to McDonald's restaurant operators earlier this year, the company announced it would phase out its all-day breakfast menu, at least temporarily. What follows are some key takeaways from that decision and some brief explanations on how similar approaches might be applied to any business facing trying times [including healthcare and, even more specifically, the SP department (SPD)]:
1. Simplify/Scale back production
As Crain's Chicago Business explained, the fast food giant’s decision was an opportunity to "streamline kitchen operations during the outbreak." Doing away with all-day breakfast likely meant increased efficiency at the drive-thru, too, since many cities at the time banned sit-down dining during the pandemic.
SP staff and other healthcare professionals can take a similar tact by identifying ways to streamline their own operations and exploring whether there are things that were historically done that consumed more than their fair share of resources within the department. If so, those might be the areas worthy of targeting first during the streamlining process. Leaders should think of task pain points that, if eliminated, would not negatively impact the quality of instruments and might, in fact, improve customer service and quality.
2. Streamline distribution
McDonald’s more limited temporary menu could create a faster customer experience, as long as the company remained confident the change wouldn’t hurt sales in the process.
Streamlined distribution is directly relatable to the healthcare segment and should be part of SP professionals’ thought process as well. Can SP leaders eliminate some decisions for their team members to allow them to focus their time, effort and energy on more important/essential tasks? During this time, many of these professionals have likely been asked to do things they have never been asked to do previously. With that added complexity, departmental leaders should ask themselves whether an opportunity exists to simplify elsewhere, without impacting quality. They may be surprised to learn that simplifying a process or task could improve employee morale while enhancing the department’s service in other areas.
3. Reinforce customer habits
Someday, today’s current troubles will lessen or altogether become a thing of the past. By streamlining policies, practices and/or procedures now, SP professionals will be better positioned to keep serving their customers most effectively and retaining (or building) a more effective interdisciplinary relationship.
This may be an excellent time for SP leaders to present and receive acceptance of changes from their partners, including departmental teammates and customers. Are there things the department does that the SP leaders and their team wish they didn't have to, or is there a better or more efficient way to do those tasks? This might also be a good opportunity to reflect on those aspects and, perhaps, even curtail some duties that aren’t serving the department or its customers well.
4. Readjust what isn’t working well
Personally, I've wondered whether all-day breakfast was a customer perk McDonald's wished it could take back. It was great marketing, but some analyses made me ponder whether it was a worthwhile business decision in the long run. If so, the pandemic delivered a silver lining: an opportunity, if not an excuse, to pull back in that area.
SP leaders should explore what simple things can be asked (perhaps to those in the Operating Room) to be done that would simplify and improve operations in sterile processing? Certainly, there are some things that likely were discussed previously (prior to the pandemic) that never came to fruition. Why not take the opportunity to raise those issues again now? We’re all having to make changes to ensure the safe delivery of care. Since everyone is already changing and, in some cases, operating outside of comfort zones, resistance could very well be lessened.
5. Control the timing
It’s worth noting that while McDonald's announced this menu change to its operators, and at least one of these operators leaked the decision to a prominent business publication, there appears to be nothing to suggest that McDonald's planned to announce the menu change publicly. This could be a lesson for healthcare as well. Again, with nearly every person facing ongoing change and understanding that “status quo” is, increasingly, no longer the norm, some changes can happen quite readily and without fanfare or drama (as long as those change won’t negatively impact quality, safety or readiness).
Seeking ways to instill positive change within the department will help set the department up for success today as well as in the future. It’s a move that could very well improve outcomes for the SPD, its healthcare customers and the patients being served.
Nicholas Schmitz is president of Schmitz Consulting LLC. He holds two master’s degrees in organization development and change management, and project management, and is a certified Project Management Professional and Lean Six Sigma Black Belt. He has served as a columnist and contributing author for the International Association of Healthcare Central Service Materiel Management (IAHCSMM) since 2016.
Sterile Processing Fundamentals: Using Metrics to Drive Efficiency
By David Taylor, MSN, RN, CNOR
This column originally appeared in the July 2020 issue of Healthcare Hygiene magazine.
Sterile processing departments (SPDs) are generally very productive units. Visitors are often surprised by the scope of responsibility and volume of work performed in a 24-hour period and have little understanding of what goes into each process. Pressuring sterile processing (SP) professionals to cut corners and increase throughput beyond what is safe, realistic, and prudent can lead to catastrophic events.
SPDs are complicated and the professionals who function within them require a great deal of knowledge and resources to manage the essential tasks appropriately. Leaders responsible for this department are being asked to improve the quality of their work, drive efficiency and do more with less as budgets continue to shrink. In addition, the number of qualified staff needed to run the department effectively is not always adequate. One way to help in these predicaments is to start running the department based upon metrics, which can provide insights into the operational efficiency of the department.
There are several ways of accomplishing this. Take for example, time-and-motion studies. They were first instituted in offices and factories in the U.S. in the early 20th century and are applicable for jobs that have repetitive tasks associated with the work, such as some of the duties in the SPD. Over the next several decades, these studies were used on a wide scale as a means of improving complex work by subdividing that work into simpler steps and sequences and eliminating redundant motion so various operations of a job can be broken down into measurable elements.1
Lean principles are also great for looking at the work being performed routinely. Lean began in the 15th century. Taiichi Ohno, a Japanese industrial engineer and businessman is considered the father of Toyota Production System. He was inspired by Lean Manufacturing in the US that was widely used by Henry Ford who worked tirelessly to eliminate waste and the efficiencies of his factories.2
By establishing benchmarks for the work performed, leaders can begin to create opportunities that allow their employees to work smarter, not harder. Wasted movement is one example. A leader can improve working conditions just by evaluating the environment and designing the workspace in such a way that allows employees to accomplish the most work with the least number of steps and movement.2
Reduce effort, improve productivity
Standard work is so important for the SPD. It serves as a fundamental platform for continuous improvement and defines the steps needed to complete a process and make corrections to that work. For example, rearranging the location of supplies and workstations can save not only steps but also add time to an SP technician’s day by allowing them to accomplish more work in the same amount of time.
Continuous workflow
Workflow patterns can have a dramatic effect on SP professionals’ ability to work efficiently. Case carts are one example. In many facilities across the country, the operating room (OR) will generally wait until it has several instrument sets or case carts before returning to the SPD (perhaps, assuming it would be easier for the SPD to reprocess in that manner). This is generally referred to as “batching,” but what the OR fails to understand is the number of responsibilities the SPD has, or the cycle times required for each step in the decontamination and reassembly process. Batching instruments and case carts increases workload, reduces the ability to reprocess instrumentation timely and generates waste. Delivering items to the SPD after each use allows those items to be reprocessed as they are received and keeps instrumentation and equipment moving throughout the day.
Windows of opportunity
Employees in one SPD spent hours walking to various parts of the hospital to collect instrumentation and durable medical equipment, and then returning those items. Not only was this a waste of time, it took a toll on those employees, creating low employee satisfaction and high turnover. The process took staff away from their core duties and required four additional full-time equivalents (FTEs). A review of this process was evaluated, and simple solutions were provided to the senior leaders. The new process proposed was to create a delivery and pick-up process that was accomplished in one step. The construction of a window -- similar to a fast food drive-thru -- would be created in a central hallway near the SPD. A representative from the various units of the hospital would deliver used equipment and receive a new like item before leaving. The savings realized allowed the SPD to reallocate the four FTEs in other areas of the department, which improved various efficiencies and the customer experience.
Walking a mile in their shoes
Another SPD employee walked 25 feet to the rack containing blue wrapping material, and then walked back to the wrapping table. The recommendation was made to move it closer to where the work was being performed. After demonstrating their efforts using a spaghetti diagram, the employees could see every step they made to and from that location. Each step was counted, timed, and calculated based on the number of instrument sets wrapped per shift. The visual representation of their work demonstrated how hard they worked in just that one aspect of their job. Once staff understood the process and were given the autonomy to decide its new location, they positioned the rack next to the wrapping station. This simple maneuver saved nearly 6,000 steps and added 90 minutes of productivity to each shift.
Conclusion
Change can be difficult. Developing processes that promote rational, logical, and efficient workflow will help the SPD bring value to their customers, allow them to accomplish more with the available resources and keep employees satisfied with their jobs.
Leaders should not be afraid to go where the work is being done and get their hands dirty, so to speak. Leaders should encourage their staff to voice their concerns and challenges – and then ask them how and what they would do to get the work done or change the process to improve the working environment. Employees are creative resources and are proud of what they do. There is no one better to provide input than those who do the work every day.
With some smart data analysis, clinical expertise and a commitment to improvement, SP leaders can create an environment that improves the health and safety of their employees and patients, meets customers’ needs and builds something of which everyone in the department can be proud.
References:
1. Time and Motion Study http://www.businessdictionary.com/definition/time-and-motion-study.html
2. Henry Ford and the Roots of Lean Manufacturing. https://www.sixsigmadaily.com/henry-ford-lean-manufacturing/
Sterile Processing Needs Better Visibility With Interdepartmental Leaders and Senior Executives
By Julie E. Williamson
This column originally appeared in the June 2020 issue of Healthcare Hygiene magazine.
Sterile Processing (SP) professionals often wish for more productive relationships with their customers, infection preventionists and risk managers. At the same time, they long for better recognition from C-suite executives and other leaders within the organization who can help ensure the SP department (SPD) and its hard-working team have what is needed to consistently tackle their roles safely, effectively and efficiently.
Among the greatest obstacles SP professionals face is getting executives to understand the needs of the SPD. This often can be attributed to two causes: lack of accessibility and poor communication, according to sterile processing director Marjorie Wall, MLOS, CRCST, CIS, CHL, CSSBB.
“SP leaders do not often have access to the C-Suite, and when they do, they’re speaking different languages,” she said, noting that executives have advanced degrees and have been working and collaborating with other executives with advanced degrees for many years. “We are starting to see an increase in SPD leaders with advanced degrees but in my experience, they are still the exception.” The result, she explained, is that many SPD leaders having critical needs and are unable to translate those needs into the business language C-Suite leaders understand. Failure to communicate effectively means many SPDs fail to get the support and resources they critically need.
Good data, good results
For SP leaders to make a big impact now and in the future, Wall advises they get comfortable being uncomfortable. That entails learning how to analyze data, build business plans and speak the language of executives. She explains that those who go into a meeting with an executive with an analysis showing how the workload has changed, how failing to match labor to the workload is resulting in increased errors/harm, what the financial impact/risk of those failures are on the Operating Room (OR) and organization and, ultimately, what the return on investment is for investing in Sterile processing staff will have a far better chance at getting the resources they need. “Never pass up a chance,” she stressed.
Over the course of her career, Wall has worked in facilities with aging sterile processing equipment that lacked the capacity to meet the OR’s high demand. In some cases, the OR expanded, adding rooms and cases, but no similar investment was made in the SPD. This resulted in equipment that was down too frequently and technicians overloading washers and sterilizers to increase throughput, which jeopardized patient safety. To overcame those challenges, Wall conducted analyses that evaluated the age of equipment, how many cycles the equipment had on it, the expected equipment life expectancy, how often the equipment was down, and how often the down equipment led to delays in care for surgery. “With this data, I have had a lot of success over the years justifying major SPD renovations and equipment investment.”
Valuable throughput data can also be attained from instrument tracking systems. Anthony Bondon, BSM, CRCST, Central Sterile Supply Manager at Sentara Lehigh Hospital in Norfolk, Virginia, routinely uses such data to show items that are consistently being used and needing to be reprocessed in the same day, which helps justify the need for more inventory and equipment. The organization also uses an operational improvement benchmarking solution that helps establish the proper volume-to-manpower ratio. Bondon said the tool has helped track volume, capture the department’s responsibilities, and ensure the department is adequately staffed to manage the duties effectively.
“I take that information to our budget meetings with all high-level executives. Before, we didn’t always get credit for bronchoscopes and some of the other devices we handled, but we can now make sure we’re getting credit for that service, which helps us justify our needs.” Facilities without computer-based tracking solutions can save their daily needs lists and tally them at month’s end.
Building a better relationship with IPs and risk managers is also critical because they look at the organization as a whole and assess changes and procedures that need to occur for patient safety and compliance outcomes, added Tony Thurmond, CRCST, CIS, CHL, central service manager at The Christ Hospital Health Network.
“We’ve invited both parties to come and spend half a day in our department to see the actual process of sending a tray to the OR, and then all the way until it comes back around for cleaning and sterilization.” This approach led to an improved relationship that paid big dividends when a risk manager saw that proper point-of-use care was not being performed in the OR and some other areas. “[The risk manager] asked us to go with them to explain how point-of-use [care] should be done. We did and we have seen a much-improved effort from all,” noted Thurmond, noting they also created a monthly compliance report for the OR and each clinic.
Knowing and sharing current standards, guidelines and key focus areas of Centers for Medicare and Medicaid Services and The Joint Commission (TJC) surveyors is another effective way to gain administrative support.
“One of the most non-compliant TJC standards relating to sterilization/high-level disinfection is the infection control standard IC.02.02.01,” noted Rose Seavey, MBA, BS, RN, CNOR, CRCST, CSPDT, retired President/CEO of Seavey Healthcare Consulting. This standard requires healthcare organizations to reduce the risk of infections associated with medical equipment, devices and supplies; therefore, surveyors will want to see that the organization has adopted evidence-based guidelines and standards, such as those published by the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative registered Nurses (AORN) and the Centers for Disease Control and Prevention (CDC), when developing infection prevention processes.
“Working with your IP and risk manager can be very advantageous in placing the emphasis on the importance of having those documents readily available to you and your staff,” Seavey said.
Julie E. Williamson is IAHCSMM's communications director/editor.
In Sterile Processing and Beyond, Desperate Times Call for Most Prudent Measures
This column originally appeared in the April 2020 issue of Healthcare Hygiene magazine.
By Julie E. Williamson
At the time of this writing, shortages of critical supplies, including face masks, respirators and other essential personal protective equipment (PPE), are further compounding the challenges. Some healthcare facilities that have been hardest hit by COVID-19 have exhausted their supply of N95 respirators and face masks and, in a desperate attempt to come up with alternatives, are relying on face shields from sterile processing department (SPD) decontamination areas; as a result, some SP professionals are being asked to disinfect and reuse their face shields – a practice that can jeopardize employee safety.
As epidemiologist Cori Ofstead, MPH, president and CEO of Ofstead and Associates, shared with the International Association of Healthcare Central Service Materiel Management (IAHCSMM), “I’m concerned that SPD personnel may be asked to use less PPE or reuse PPE. If that happens, they should sit down with stakeholders and figure out how they’re going to decontaminate the PPE before reuse. Anything used in manual cleaning is highly contaminated and should not be reused, unless it can be cleaned and disinfected or sterilized in ways that do not compromise the materials. Vendor consultations may be necessary for this.”1
In the SP realm, COVID-19 has provided some essential teachable moments. For starters, there is good news: with practice due diligence, the virus can be eliminated by high-level disinfection and sterilization.2 As Ofstead explained, the risk is no higher than it has always been with other serious pathogens, and everyone will be safe if they follow the practices needed to prevent exposure to HIV, hepatitis, bacteria and fungi.1
Currently, OSHA recommends following SARS disinfection practices (see section D-10) for environmental areas contaminated with COVID-19. Note: The CDC advises the use of EPA-registered chemical germicides that provide low- or intermediate-level disinfection for SARS during general use (surface and noncritical patient-care equipment) because these products inactivate related viruses with similar physical and biochemical properties. Further, the CDC's Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 provides information on germicides’ efficacy against coronaviruses.
SP professionals should always treat every device they handle in the decontamination area as high risk and potentially contaminated with microorganisms that can cause devastating, if not fatal, infections. Every day when SP technicians enter the department, they must understand that any device that has not been thoroughly and properly cleaned cannot be effectively sterilized. Also, when manually cleaning any instrument, the devices must be fully submerged underwater to prevent splashing and aerosolization.
Where COVID-19 is concerned, recent studies have shown the virus can survive for hours or even days on surfaces that aren’t cleaned or disinfected.3,4 Again, even taking the novel virus out of the equation, it’s essential that – in addition to instruments themselves -- all work areas and transport bins must be routinely, thoroughly and properly disinfected in accordance with the manufacturer’s instructions for use and standards-based facility policies and procedures.
Proper training and competency testing to ensure all SP technicians are following correct practices for handwashing and donning/doffing of personal protective equipment (PPE) is imperative and must be performed on an ongoing basis. Unfortunately, both practices tend to be lacking.
“We have never been to an institution where all of the reprocessing technicians were wearing the proper PPE in the correct way,” notes Ofstead, adding that technicians also rarely use proper technique when taking off PPE, which could expose them to COVID-19 and other pathogens.
Masks and face shields are often inappropriately worn, perhaps due to lack of hands-on training or the uncomfortable environment in the decontamination area, which may make their removal tempting. The top must pinch snugly on the nose to prevent it from migrating down and exposing the nose or mouth, Ofstead explained. She warned against allowing the mask to dangle around the neck and then pushing it back up for use throughout the day – regardless of how uncomfortable those face coverings may be.
It’s also worth noting that even though many SP professionals may be seeing their workload diminish due to COVID-19-related cancellations of elective surgeries, their SPDs will be busier than ever once life begins to return to normal, restrictions lift and hospitals and surgery centers start rescheduling those procedures. The need for all SP professionals to practice due diligence and avoid taking any shortcuts will be greater than ever. It’s a message that all should take to heart every day of the year, for every case and every instrument.
Julie E. Williamson, BA, is IAHCSMM’s communications director/editor.
References:
1. IAHCSMM. Conversation with Cori Ofstead Regarding COVID-19. IAHCSMM Insights. March 25, 2020.
2. OSHA. COVID-19 Control and Prevention, Interim Guidance for Most U.S. Workers and Employers of Workers with Potential Occupational Exposures to COVID-19: Environmental Decontamination. https://www.osha.gov/SLTC/covid-19/controlprevention.html#health
3. CDC. Public Health Response to COVID-19 Outbreaks on Cruise Ships – Worldwide, Feb-March 2020. Morbidity and Mortality Weekly Report. https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e3.htm?s_cid=mm6912e3_w
4. NIH. New Coronavirus Stable for Hours of Surfaces. March 17, 2020. https://www.nih.gov/news-events/news-releases/new-coronavirus-stable-hours-surfaces
Certification Can Improve Sterile Processing Quality and Patient Outcomes
By Rose Seavey, RN, BS, MBA, CNOR, CRCST, CSPDT
Editor's note: This column originally appeared in the March 2020 issue of Healthcare Hygiene magazine.
Certification is a notable and increasingly important achievement for any healthcare discipline. Its attainment is one that not only benefits the individual who attains it, but also their department and facility and, above all, the patients being served. Increasingly, employers view professional certification as a way to evaluate whether an individual has the depth of skills and knowledge required for them to perform successfully, confidently and safely in a particular role. Employers want to hire the most qualified individual who will make a positive contribution to quality patient care, and they may determine that certified individuals possess that “extra something” the facility needs and seeks.
Most healthcare facilities are certified as an organization. Passing an accreditation survey, such as from The Joint Commission, for example, is a healthcare organization’s process of certification. The healthcare industry sees certification as an important aspect of providing safe patient care; therefore, it’s understandable that more facilities are holding in high esteem individuals who are certified in their respective roles. Attaining and maintaining certification helps ensure safe quality care through process improvement and risk reductions based on knowing, understanding and following evidence-based standards. This is certainly important for those responsible for reprocessing medical and surgical instrumentation.
The Association for the Advancement of Medical Instrumentation (AAMI) recommends all personnel performing reprocessing activities be certified within two years of employment and then maintain that certification throughout their employment. AAMI also recommends that those who manage supervisory functions be adequately prepared for this responsibility through education, training and experience, and have minimum recommended qualifications that include successful completion of a sterile processing (SP) management certification examination.
Patients rarely (if ever) meet SP professionals; however, their effective care and treatment nonetheless hinges on these individuals’ performance in their various roles within the department. Patients and healthcare customers such as those in the operating room, emergency department, labor & delivery, and endoscopy, to name a few, rely on SP professionals to provide safe, clean, sterile and well-functioning instrumentation. They also count on SP professionals to follow current evidence-based best practices, as well as the latest standards, guidelines and instructions for use.
Certification helps support these expectations and lends assurance that SP professionals have the essential knowledge and skills needed for managing critical departmental responsibilities safely, effectively and consistently. Continuing education requirements for SP professionals to maintain their certification status further helps ensure that they can stay on top of ever-evolving instrumentation, technology, standards and best practices to keep the department functioning at its best.
The International Association of Healthcare Central Service Materiel Management (IAHCSMM) is a longstanding and dedicated advocate for SP professionals. This is evident in IAHCSMM’s advocacy roles that include promoting education and legislation at the individual state level. The Association’s highest legislative priority is to ensure there are certified SP technicians in every healthcare facility in every state. Although only four states (Connecticut, New Jersey, New York and Tennessee) currently require certification of SP technicians, IAHCSMM is actively pursuing legislation in other states.
Facilities in states that do not currently require certification of SP technicians can and should make certification a requirement within their own organization. Managers or supervisors working in a state or facility that does not yet require certification are highly encouraged to work with their facilities’ human resources and administrative professionals to educate them on the many merits of SP certification and why it would benefit the facility to require certification of SP technicians as a condition of employment.
Specifically, certification of SP technicians can assist with the following:
• Developing the basic level of understanding and knowledge for reprocessing medical devices
• Providing internationally recognized, competency-based, measurable standards
• Offering practice consistencies and standardization
• Adding a professional element to the department
• Building self-esteem, confidence and authority to the individual who holds the certification(s).
It is equally important to note that SP professionals needn’t wait for their state or facility to require certification. Instead, individuals are encouraged to make a personal commitment to become certified. Sterile processing is a profession, not just a job. It’s also a profession that has a real and direct impact on patient care, safety and outcomes. Every patient deserves quality-focused, dedicated and well educated/well skilled reprocessing professionals who are committed to ongoing knowledge advancement and skill set development. When an individual makes a personal commitment to acquire certification and then maintain that certification through continuing education, it helps demonstrate their dedication to quality and professionalism.
For more information about IAHCSMM’s certification offerings, visit: www.iahcsmm.org/certification.html.
Rose Seavey, RN, BS, MBA, CNOR, CRCST, CSPDT, is the retired president/CEO of Seavey Healthcare Consulting and former director of the SPD at the Children’s Hospital of Denver.
Current Standards Vital to Sterile Processing Success: Does Your SPD Have the Latest?
By Julie E. Williamson
This column originally appeared in the February 2020 issue of Healthcare Hygiene magazine.
Sterile Processing departments (SPDs) functioning in the absence of the latest industry standards, guidelines and recommended practices are at risk of a multitude of negative outcomes, not the least of which can include increased risks to patients and healthcare workers; survey deficiencies, citations and fines; and potentially devastating damage to the facility's reputation.
It's a message echoed by many experts -- from SP veterans, consultants, and surveyors from the Joint Commission (TJC) and Centers for Medicare and Medicaid Services (CMS), to litigators, representatives from healthcare associations and government agencies, and more. Unfortunately, many facilities lack these critical resources, and many may not even be aware.
Sometimes, SP professionals don't have any version of certain standards; others may only have outdated versions – such as an older version of ANSI/AAMI ST79, Comprehensive guide to steam sterilization and sterility assurance in health care facilities, instead of the most current 2017 version. Having current standards readily available to employees is not just extremely important for the SPD, but for the entire facility. ST79 is a comprehensive document with valuable information for the SPD, operating room, infection prevention, safety, risk management, and engineering/facilities management. ANSI/AAMI ST79: 2017 is the go-to reference for steam sterilization in all healthcare facilities and is applicable regardless of sterilizer size or facility size1. Developed by sterilization and manufacturing professionals ST79:2017 can help SP professionals:
• Support safety at every step for processing medical devices;
• Follow proper sterilization practices of processed items across multiple facility departments
• Understand the complete life cycle of sterilization processing - from managing temperature and humidity to processes for the decontamination area
• Substantiate the need for equipment upgrades to meet federal guidelines that reduce potential citations
• Support the delivery of properly processed medical devices critical to optimizing patients’ health
• Guide personnel toward desirable performance objectives1
Its availability and use will also help facilities stay in compliance with accrediting bodies such as The Joint Commission and the Centers for Medicare and Medicaid Services. AAMI notes that it is the document by which surveyors are being trained; therefore, surveyors will be looking to ensure the facilities they inspect are well versed on that document and have it readily available to staff members.
Limited budgets are often to blame for the lack of standards in the SPD. To help SP professionals petition their facilities for the most current standards, guidelines and recommended practices needed for quality customer service and patient safety, the International Association of Healthcare Central Service Materiel Management (IAHCSMM) drafted a Standards Value Letter, a free, customizable template that SP managers can share with administrators and other executives.2
The Standards Value Letter lists the most current standards, Technical Information Reports (TIRs) and guidelines facilities should have available to all individuals responsible for SP functions. These include the following from the Association for the Advancement of Medical Instrumentation (AAMI), the Association of periOperative Registered Nurses (AORN) and the Centers for Disease Control and Prevention (CDC):
1. ANSI/AAMI ST79: 2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities
2. AORN Guidelines for Perioperative Practices, 2020
3. ANSI/AAMI ST58:2013, Chemical sterilization and high-level disinfection in healthcare facilities (Note: This document is currently under review)
4. ANSI/AAMI ST41: 2008/(R) 2018, Ethylene oxide sterilization in healthcare facilities (for facilities using ethylene oxide)
5. ANSI/AAMI ST91:2015, Flexible and semi-rigid endoscope processing in health care facilities
6. ANSI/AAMI ST90:2017, Processing of health care products - Quality management systems for processing in health care facilities
7. AAMI TIR 68:2018, Low and intermediate-level disinfection in healthcare settings for medical devices and patient care equipment and sterile processing environmental surfaces
8. AAMI TIR 67:2018, Promoting safe practices pertaining to the use of sterilant and disinfectant chemical in health care facilities
9. AAMI TIR 34:2014, Water for the reprocessing of medical device
10. AAMI TIR 63:2014, Management of loaned critical and semi-critical medical devices that require sterilization or high-level disinfection
11. CDC Guideline for Decontamination and Sterilization in Healthcare Facilities, 2008
The CDC Guideline for Decontamination and Sterilization in Healthcare Facilities, 2008, is available free of charge on the agency’s website (https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf). Note: This version was updated in May 2019 and all SPDs should have that version on file for their SP professionals. There is a charge for the other documents (AAMI and IAHCSMM members receive discounts); however, budgeting for these documents is essential given healthcare organizations’ need and desire to provide the very best patient care and quality customer service. Failing to have current standards on file in one’s department could prove costly for facilities if adverse events occur, such preventable infections or injuries to patients or staff, or subsequent litigation.
Diligent adherence to the latest standards and best practices will help drive patient and employee safety, advance infection prevention efforts, and keep exemplary customer service a top priority.
To access the IAHCSMM Standards Value Letter, visit: https://www.iahcsmm.org/resource-documents/iahcsmm-standards-value-letter.html
Julie E. Williamson, BA, is IAHCSMM’s communications director/editor.
References:
1. ANSI/AAMI ST79:2017. https://www.aami.org/productspublications/ProductDetail.aspx?ItemNumber=1383
2. IAHCSMM Standards Value Letter. https://www.iahcsmm.org/resource-documents/iahcsmm-standards-value-letter.html
Instructions for Use, Inadequate Support Pose Medical Device Cleaning Challenges for Sterile Processing
By Damien Berg, CRCST
This Perspectives column originally appeared in the January 2020 issue of Healthcare Hygiene magazine.
Sterile processing (SP) professionals face many challenges in today’s complex, ever-evolving instrument processing landscape. One of the greatest obstacles involves complying with manufacturers’ instructions for use (IFU) for cleaning/disinfecting medical devices and, at the same time, dealing with time and resource constraints, difficult conditions in the decontamination area, and more.
Whether a facility or organization calls the department “Sterile Processing,” “Central Service,” “Central Sterile Supply” or another name, the department’s primary responsibility remains the same: reprocessing a wide array of medical/surgical devices that often include complex orthopedic instruments, sophisticated power equipment, narrow-lumened laparoscopic devices, and ever-challenging flexible endoscopes. Each of these devices has specific, often confusing and even conflicting cleaning instructions. Frontline SP technicians must navigate these IFU while also facing increasing and, sometimes, unrealistic demands from the operating room (OR) or another end-user department.1 Often, SP professionals field requests to turn instruments around more quickly than is safe or prudent, and those requests often don’t align with manufacturers’ IFU or even industry standards. If the cleaning process is rushed or any missteps occur, high-level disinfection (HLD) and sterilization will be negatively affected, and patient safety becomes jeopardized; it’s a point underscored in numerous studies, including a recent joint study by Ofstead & Associates that was published.2
When it comes to the confusion and frustration associated IFU compliance and meeting the requirements of The Joint Commission (TJC), the hospital and SP leadership must understand the common challenges that can cause the organization to become noncompliant during surveys. In 2017, 72 percent of surveyed hospitals and critical access hospitals were found to be noncompliant with TJC’s infection control standard, IC.02.02.011;3 the standards require hospitals to reduce infection risks associated with medical equipment, devices and supplies, primarily as it related to steps of HLD and sterilization. This high degree of noncompliance led TJC to revise the standard to include more specific scoring that focuses on areas of highest risk. The changes took effect Sept. 1, 2018.
TJC will continue to score IC.02.02.01 as noncompliant whenever manufacturer instructions are not followed. This includes following the IFU to the letter in the decontamination area (including soak time, sonic time, and manual cleaning and automatic cleaning steps). It is not only critical for SP professionals to understand these tasks and details but also to be able to speak to the IFU and how the medical device is cleaned and disinfected prior to HLD and/or sterilization.
Unfortunately, challenges abound because the IFU are not always clear or easy for the frontline SP technician to understand. Technicians are also frequently pressed for time and often lack adequate instrumentation inventories to meet turnaround time requests from the OR and other patient-care areas. Beyond that, some technicians/facilities don’t have access to the correct processing equipment – or they may lack enough equipment to meet the instrumentation and turnaround demands.
All these gaps create the perfect conditions for failure. The Association for the Advancement of Medical Instrumentation (AAMI) has been working on its technical information report, TIR12: Designing, testing, and labeling reusable medical devices for reprocessing in healthcare facilities, which will help manufacturers standardize their cleaning instructions so the end user can better understand and follow them. Developing this document required significant time as it involved the review of more than 250 IFU and explored the commonalities and complexities of cleaning. In doing so, the end goal is to provide manufacturers with a blueprint and understanding of what the modern SPD entails and how it operates, so manufacturers can design their devices for improved cleanability and develop realistic, easily understood cleaning and disinfection instructions that can be performed effectively in today’s instrument processing areas/departments.
Having regulatory agencies, standards-making bodies and manufacturers of complex medical devices on the same page in their understanding of the urgent need for safer, easier to clean devices and easier to improved cleaning instructions will bode well for compliance and patient safety. It is also essential that hospitals and other healthcare facilities ensure they are supporting reprocessing areas with appropriate staffing levels; providing proper and ongoing training; ensuring instrumentation inventories are adequate to meet procedure volume; and committing to providing enough modern processing equipment to meet the needs of today’s modern OR. Technology in the procedural areas is becoming more complex and sophisticated in order to provide the best and safest patient care; therefore, it is imperative that those who perform instrument cleaning and sterilization have the proper tools and training to ensure that those devices are clean, sterile and ready when needed.
Today’s healthcare environment resides in a world of risk assessments and a simultaneous push for cost containment; however, it is critical that healthcare organizations do not overlook the common factors that increase their own risks and, most importantly, the patient’s. Among the greatest risks are the human factors associated with cleaning and disinfecting surgical instruments and complex medical devices. This should be considered the foundation of any successful clinical outcome.
Highly skilled, experienced and well-trained clinicians are the frontline of patient care, but the supporting work of reprocessing professionals ensures the clinical team not only has the correct medical devices when they are needed, but also that each device is properly functioning, clean, disinfected and sterile. This vital need can only be met when SP professionals are adequately supported in their roles. This involves ensuring that these professionals not only possess a proper understanding of all reprocessing steps, but are also provided with clear, easy to understand IFU and enough instrumentation and equipment to perform those IFU consistently and precisely as written.
References:
1. The Joint Commission. Joint Commission Revised IC Devices Standard. October 1, 2018. https://www.reliasmedia.com/articles/143328-joint-commission-revises-ic-devices-standard.
2. Ofstead et al. Endoscope Preprocessing: Current Practices and Challenges in the Field. PROCESS. July/Aug 2019. International Association of Healthcare Central Service Materiel Management.
3. ECRI Institute. If It’s Not Clean, It’s Not Sterile: Reprocessing Contaminated Instruments. April 11, 2017. https://www.ecri.org/components/PSOCore/Pages/e-lert041117.aspx.
Terminal Cleaning in the SPD: A Critical Step in Infection Prevention
By Julie E. Williamson
This column originally appeared in the January 2020 issue of Healthcare Hygiene magazine.
Ensuring that surgical instruments are cleaned, disinfected/sterilized and safe for patient use involves many steps, processes, check and balances. One critical step is making certain surfaces are properly disinfected and other housekeeping tasks are routinely performed in accordance with guidelines, standards and facility policies and procedures. Sterile processing departments (SPDs) must be routinely cleaned to minimize microbial population; the cleaner the work area(s), the more likely the items prepared in the SPD will be safe for use in a sterile environment.1
Reaching that important goal is often a shared responsibility of Environmental Services (EVS) personnel and Sterile Processing (SP) professionals, with facilities determining the appropriate cleaning tasks and schedules for each. While EVS often bears much of the actual terminal cleaning responsibility, SP professionals often routinely clean sterile storage cabinets, carts and racks.1 SP professionals must also ensure they and their colleagues don’t further contaminate work areas or engage in practices that can lead to cross-contamination or contribute to healthcare-associated infections (HAIs).
Contaminated items and surfaces, including door handles, faucets, light switches, keyboards, telephones, work tables and more, can transmit infection-causing bacteria called fomites; therefore, work areas should be routinely and thoroughly cleaned – and SP professionals must aim to minimize the amount of contaminants throughout the departments. The presence of dust, lint and bacteria on devices that need to be high-level disinfected or sterilized may negatively impact those critical processes, and particles or bacteria that make their way into a sterile set may enter the patient’s body and cause an infection.1
Microorganisms can survive on surfaces for long periods of time. For example, Clostridium difficile can survive from weeks to months and Staphylococcus aureus can survive for months on a dry surface.2 If surfaces are not properly cleaned and disinfected, these organisms can become a continuous source of contamination. A study of 23 acute care hospitals found that, on average, only 49 percent of surfaces that were believed to have been properly cleaned actually were.3
Eating or drinking should never be performed in SPD work areas (on both dirty and clean sides). Beverages may spill, leading to potential contamination of devices, surfaces, reference books and other items, and food crumbs can attract disease-causing insects or rodents to the work area. Additionally, food oils and residues can be passed from hands to instruments and other surfaces, which may impede effective cleaning and sterilization.
All fixtures and furnishings in the SPD must be made of materials that can be cleaned and disinfected on a regularly scheduled basis. Sterile storage areas may have either open racks or closed cabinets; however, closed cabinets are best for high-traffic areas. Open shelving should have a solid bottom, so items stores on lower shelves are protects from contaminants during housekeeping tasks.1 Corrugated cardboard boxes and external shipping containers should not be allowed in storage areas or other parts of the SPD because they may harbor microorganisms and introduce other contaminants to the areas.4 If anti-fatigue floor mats are used in the department, they must be designed to withstand daily cleaning and disinfection and should be discarded when they show visible wear or breakdown that can inhibit proper cleaning or introduce particles into the environment.
Terminal cleaning and disinfection of the SPD should not be performed while instruments are being cleaned and sterilized/high-level disinfected. Also, clean-to-dirty flow should always be followed, beginning with sterile storage, then moving to preparation and packaging and, finally, to the decontamination area to reduce the risk of spreading contaminants from “dirty” areas to “clean” areas of the department. Cleaning should also be approached with a “top-down” method and some facilities may also adopt a left-to-right method to ensure no items are missed during the cleaning process.
Floors should be cleaned at least daily with a damp mop; dry sweeping or mopping should not be done because dust and other contaminants will become airborne and can land on instruments, work tables and other surfaces.1,4 Contaminants on sterile packages can fall onto package contents upon opening and jeopardize patient safety. Separate and dedicated cleaning equipment, such as mops and buckets, should be used for the decontamination area, which is the dirtiest area of the SPD. This cleaning equipment should never be used elsewhere.
Lighting fixtures or their covers and air vents should be cleaned at least every six months or as needed.1 Note: This task is typically performed by EVS or plant maintenance, as opposed to SP professionals. Horizontal work surfaces should be cleaned daily or, preferably, at the end of each shift. Walls, cabinets, shelving or other surfaces should also be regularly cleaned, at an interval determined by the facility, and as needed. Checklists and audits can be helpful for ensuring all areas of the department are properly cleaned and at the correct intervals.
Any individual with cleaning responsibilities requires targeted, ongoing training to ensure departmental policies and procedures are consistently followed; this includes ensuring that proper personal protective equipment is being used during departmental cleaning, proper chemical dilution rates and contact times are being followed in accordance with manufacturers’ instructions for use, and surface compatibility is taken into consideration.4
Julie E. Williamson, BA, is communications director/editor for the International Association of Healthcare Central Service Materiel Management (IAHCSMM).
References:
1. International Association of Healthcare Central Service Materiel Management. Central Service Technical Manual, Eighth Edition. Chapter 6. 2016.
2. Kramer, et al. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BioMed Central. 2006.
3. Carling, et al. Identifying Opportunities to Enhance Environmental Cleaning n 23 Acute Care Hospitals. Infect Control Hosp Epidemiol. January 2008, 29(1): 1-7.
4. Huber L. Surface Disinfection and Departmental Housekeeping in Central Service. CIS Lesson Plan 253, Communiqué. Jan./Feb. 2016. International Association of Healthcare Central Service Materiel Management.
Proactive Device Care Helps Eliminate Biofilm Threat, Aid Cleaning Process
By Julie E. Williamson
This column originally appeared in the December 2019 issue of Healthcare Hygiene magazine.
Preventing contaminated instruments from being used in a subsequent procedure should be a top priority for every professional in sterile processing (SP), the operating room (OR) and other end-user departments. Any bioburden that remains on an instrument can cause devastating, potentially deadly infections if that contaminated device or piece of equipment is used on another patient. It’s important that all caregivers and reprocessing professionals understand that instrument contamination can’t always be detected with the naked eye, and that proactive instrument care is a shared role that must be consistently and diligently performed.
Biofilm is an often-invisible threat to patient safety and its presence can rapidly progress to a significant problem if not promptly and properly addressed. Biofilm is a collection of microorganisms that attaches to surfaces and itself to form a colony1 that then produces a protective gel matrix on device surfaces. This matrix cannot be easily penetrated with detergents and disinfectants – an especially important fact considering instruments that are not thoroughly cleaned cannot be effectively high-level disinfected or sterilized.
Wava Truscott, BS, MBA, PhD, of Truscott MedSci Associates, explained there are six stages of biofilm development and each stage is increasingly difficult to eradicate.2 Stages 1 and 2 can be easily removed, however, any biofilm that remains quickly sends out a signal to multiply further. Stages 3 and 4 are even more resilient and resistant to eradication, and Stage 5 is very mature biofilm that is especially difficult to destroy. Stage 6, the highest level, is when biofilm becomes hardened on devices such as endoscopes that have undergone reprocessing. In this stage, the biofilm builds upon itself and forms what Truscott describes as a matrix fortress.
“When bacteria determine that the surface they landed on has an organic food supply and is physically a good place to construct a biofilm, they signal other bacteria to join them. The favorable response also triggers its 'appendages' to attach to the surface and to other bacteria, while physically enhancing the strength of the attachments’ grip," she explains, adding that endoscopes, catheters and narrow tubes and channels are just some of the places where bacteria can hide and quickly multiply.
Biofilm is designed to survive and thrive; therefore, proper proactive instrument care and treatment is vital for preventing its development and proliferation. Although instrument and equipment cleaning, high-level disinfection (HLD)/sterilization takes place in dedicated reprocessing areas, point-of-use care is necessary for preventing blood, tissue, secretions and other types of bioburden from drying on surfaces. Dried bioburden makes cleaning in the SP department and other designated reprocessing areas far more challenging.
Point-of-use treatment should begin immediately following the procedure (and in the location where the procedure occurred). This treatment involves wiping gross blood and bioburden from instruments and keeping devices moist with an approved wetting agent (an enzymatic or moisturizing gel or spray, for example), all the way through transport to the dedicated decontamination area. If moisturizing products are unavailable, instruments should be covered with a water-moistened towel (saline should never be used, however, because it can corrode or otherwise damage instruments). Note: A single surviving bacterium can multiply to 2 million in just seven hours; therefore, it is imperative that devices be transported to the decontamination area as soon as possible following the procedure.2
The latest industry standards and guidelines, including those from AAMI, AORN and AST recommend point-of-use instrument care to remove gross debris and aid the cleaning process. ANSI/AAMI ST79:2017, Comprehensive guide to steam sterilization and sterility assurance in health care facilities, Section 6.3.1, states that instruments should “be wiped throughout the surgical and invasive procedure, as needed, with sterile moistened surgical sponges to remove gross soil. Cannulated or lumened instruments should be irrigated with sterile water, as needed.”
Once contaminated devices enter the decontamination area, proper cleaning tools and application are essential. Cleaning brushes should be neither too small (which can scratch or gouge instruments and create a place for microorganisms and bioburden to hide and thrive) or too large (which prevents the bristles from cleaning properly). Using proper pressure while brushing is also key because too rigorous brushing can damage instrument surfaces and too-gently brushing can prevent proper removal of bioburden. Water quality also factors into reprocessing outcomes. Contaminated water can cause infection and water with high mineral concentration or the presence of organic matter can cause buildup on instruments that can make disinfectants and sterilants ineffective. Truscott reminds us that faucet aerators also provide an opportune location for microorganisms and biofilm to hide and flourish.2
Facilities that fail to follow the latest industry standards and guidelines – and manufacturers’ instructions for use – are not only jeopardizing patient safety, but also increasing the odds for citations and fines from surveyors such as those from the Joint Commission and Centers for Medicare and Medicaid Services. Increasingly, surveyors are well versed on the latest standards and guidelines and are looking to ensure that facilities are following them. Internal policies and procedures are also being carefully reviewed; therefore, if a point-of-use care policy is in place, surveyors will want to see that it is being consistently followed.
Julie E. Williamson, BA, is IAHCSMM’s communications director and editor.
References:
- International Association of Healthcare Central Service Materiel Management. 2016. Central Service Technical Manual, Eighth Edition.
- Truscott W. April 2019. Biofilm Housing Development: Work Surfaces, Devices, Cleaning Equipment. Session at the 2019 IAHCSMM Annual Conference.
The Tip of the Iceberg: It’s Not Just Goshen
By Hank Balch
This column originally appeared in the December 2019 issue of Healthcare Hygiene magazine.
On Nov. 18, 2019, hospital administrators at Goshen Health in Goshen, Ind. notified nearly 1,200 surgical patients that they may have exposed to hepatitis B, hepatitis C and HIV due to improperly processed surgical instruments. 1 The story itself is one that we've heard again and again in recent years, from places like Seattle Children's Hospital, Detroit Medical Center, and Porter Adventist Hospital in Denver.
While the locations for these quality breakdowns change, the overarching script does not. Some process was not followed, some step in Sterile Processing was not taken, and now thousands of patients are given the news that instead of healing them, their hospital visit may have infected them with a deadly virus. Not the kind of news any patient ever deserves. Unfortunately, most patients have no idea how systemic these challenges really are.
As frightening as this news is for the public at large and surgery patients in particular, inside the sterile processing (SP) industry we are not surprised when we see a headline like this hit the evening news. In fact, many of us are surprised that we don't see more of them. Discussions around non-compliance for point-of-use cleaning, challenges around manual cleaning protocols, breakdowns in automatic cleaning equipment, and staff competency concerns are constantly discussed during our national annual meetings and local seminars. Research is regularly being presented via whit papers, industry magazines, and posters that highlight serious shortcomings related to current cleaning, disinfection, and storage practices in the field.
For SP consultants who visit multiple facilities a month across the country, there is no question that the kinds of quality headlines we see from hospitals like Goshen are far more common than the public is aware. Simply put, we all know that we are just beginning to scratch the surface of the real depth and breadth of these infection control risks. While the mainstream media reports on the occasional tip of the iceberg that floats on the surface, there is a massive problem lurking just underneath that has the attention of many SP professionals, microbiologists and regulatory agencies.
When specific process breakdowns like this are identified in hospitals, there is an immediate rush to calm public fears, get accurate information out to media agencies, and try to explain how something like this could happen in SP. In fact, the CDC has an entire resource page dedicated to walking facilities through this notification process in a transparent, yet controlled manner.
One of the central phrases from the CDC resources instructs hospitals to tell patients, “We believe the risk to be extremely low.” If you closely review the communications from the hospitals listed at the beginning of this article, and other examples of surgical sterilization problems, you will see this refrain used again and again. While the comparatively low risk of exposure is true enough in a purely statistical sense, when we hear interviews from the patients who receive these notifications there is near unanimous concern, fear, and anger. One patient from the Goshen example said it this way, “I was mad, I was really, really mad because when you tell somebody that they could be at risk for something like that, it not only involves you, it involves your family, your significant other. I mean I have grand kids and kids. I have a life."2
This patient feedback is a far better indicator for what the public finds value in knowing about how their surgical care is delivered to them. Patients who have been notified of an infection control breach do not care about cold, dry statistics from some government agency. They want to know why this happened in their town, during their surgery, and if the results of their test is going to change the rest of their life.
So how do we bridge this disconnect between tremendous ongoing quality struggles in SPDs around the country, and public awareness of the situation before it leads to a wide-scale patient notification scenario? One of the best opportunities before us is to bring our internal industry conversations out into the public arena. This will mean a pivot from talking primarily to ourselves about ourselves, to talking and educating a public who has very little understanding of the current state of medical device reprocessing.
A great example of this type of public facing approach can be seen in the work of Aakash Agarwal, PhD, who has conducted recent media interviews and publications around contamination concerns with surgical implants. Through these platforms, and other social media outlets, Agarwal is engaging with this topic in the public sphere, where potential patients can encounter and respond to the content from an educational perspective. This is just one of a hundred different topics that touch the Sterile Processing industry which could be more actively and transparently discussed, with the goal of stirring up awareness of and support for additional resources to find real solutions for the challenges that currently plague us.
The longer we respond to situations like Goshen Hospital as if they were the rare exception, instead of an ominous symptom of a deeper problem, the larger the risks grow to see more patients receive notifications of potential exposure. Instead, we should pull back our industry curtains, and let the light of public transparency melt the quality iceberg in our path.
Hank Balch is an internationally recognized thought leader in the sterile processing industry, as well as podcast host, and founder of Beyond Clean.
References:
- https://wsbt.com/news/local/close-to-1200-patients-at-goshen-hospital-may-have-been-exposed-to-infectious-disease
- https://wsbt.com/news/local/it-scared-the-heck-out-of-me-goshen-hospital-patient-says-shes-worried-for-her-future
IFUs: The Challenges and Opportunities for Compliance
By Kelly M. Pyrek
This article originally appeared in the November 2019 issue of Healthcare Hygiene magazine.
Healthcare Hygiene magazine spoke with Susan Klacik, BS, CRCST, CHL, CIS, ACE, FCS, clinical educator with the International Association of Healthcare Central Service Materiels Management (IAHCSMM), about the importance of following manufacturers’ instructions for use (IFUs) as part of the overall strategy for proper medical device reprocessing and upholding patient safety.
HHM: Talk to us about the importance of following IFUs.
SK: IFUs share with us the key details about how to properly process a medical device or instrument. IFUs are developed based on the scientific validation on that specific medical device; the devices are soiled with a certain kind of soil that is reflective of soil it will be exposed to. There are measurements taken around the level of soil and how this soil is eradicated from the device, and how exactly to do so. Testing labs determine the cleaning and disinfection protocol, and manufacturers list out these steps for removing the soil. IFUs are becoming so detailed now, that manufacturers are even indicating what kind of brushes to use. This is especially good information, especially if a facility is going to buy a new medical device – that’s the information we need up front so that when we have the medical device in front of us, we don’t say, ‘Oops, we don’t have the right brush on hand.”
HHM: With more detailed IFUs comes new challenges of compliance, right?
SK: Right, and the IFUs do change, compounding the challenge even more. We must keep them updated but it is a challenge because some of them are very, very detailed and they are difficult for techs to follow. We often must use our critical thinking skills. For much more complex medical devices, we really must follow IFUs to the letter because that’s where all the validation information is contained. Labs inoculate the device, and they know exactly what it takes to clean it and sterilize it, and if we aren’t following the IFUs, we can harm patients. Also, we risk damaging the device if we don’t follow the IFUs. The bottom line is, we don’t want to have any debris remaining on or in the device because we don’t want to give our patients infections, and that’s why we must follow the IFUs.
HHM: Can healthcare facilities do a better job of explaining these imperatives and improving techs’ comprehension of the science?
SK: The challenge around educating continues. It’s always best to explain the “why” behind why we do what we do; because then CS/SPD personnel perform their jobs better when they understand more about the “why.” So, I think the “why” is very important. Techs must understand that validation is based on the science, which drives everything that we do.
HHM: Is the onus on the manager?
Yes, sterile processing leaders are responsible for the education and training of their personnel. We need to hold a lot of in-services as new medical devices and instruments come into the healthcare facility, and the manufacturer must help in-service staff as well. These new devices and instruments shouldn’t be used until the sterile processing staff is in-serviced, period. The sterile processing leader can also identify their problem items and start in-serving techs on those IFUs. Many manufacturers offer online resources, as does IAHCSMM, which provides instructional resources including webinars and now podcasts. Time is also a factor because we are so busy.
HHM: Could providing techs with feedback about HAIs and facility infection rates could help?
SK: We need to educate them around why we must process based on scientific validation – it’s not just infections, it’s overall patient safety.
HHM: Has there been any progress on removing the barriers to improvement in sterile processing?
SK: The biggest barriers remain, such as limited processing capacity due to shortages in staff, tools, equipment and resources. Or unrealistic turnaround times. For example, some IFUs mandate a 20-minute soak time, but the operating room needs it faster than that. We must get with the surgical services department and educate them around the numerous steps in the average IFU, and that we need adequate time from when the device or instrument first lands in the decontamination department to when it gets packaged and sent back to the OR. We must better educate our customers in the OR so that they are not pressuring us. Maybe the healthcare institution needs to buy more equipment. When IFUs change, or involve things like robotics, a lot of hospitals increase their instrument inventory because it extended their processing time. We need to communicate clearly with our OR customers because they don’t understand all the complicated steps required to process a medical device or instrument.
Researcher Cori Ofstead and her team conducted a study that showed it takes about 74 minutes to clean a scope, yet sterile processing is continually pressured to deliver in half that time, despite complicated IFUs. We must educate around IFU compliance and emphasize that the OR must schedule patients differently or buy more scopes. We must work with our OR customers better for them to understand what is involved in processing these increasingly complex medical devices and instruments.
HHM: Speaking of Cori Ofstead; her recent survey of IAHCSMM members revealed numerous challenges that persist in CS/SPD.
SK: We are moving toward improvement, we just aren’t getting there as fast as I wish we would and should. Barriers to better practice are not being entirely eliminated and we have a ways to go, but I don’t want to discourage anyone because we have worked so hard to get where we are. We need to keep going; to me, the survey results tell me we are making progress but not as fast we wish we could go.
HHM: Are manufacturers realizing they can help make devices that are easier to process, and IFUs that might be easier to understand, as part of the solution?
SK: The dial is moving slowly; there are some companies that are providing better resources for sterile processing. We’re moving slowly but we’re not quite there yet. The problem with IFUs is that some lack information. In most cases, it’s the older ones that lack critical information we need, while others are so detailed that they are almost impossible to follow completely, so we have both ends of the spectrum. In 2015 the FDA published their latest labeling guidance and that provided some help. We have the FDA’s ear and they are trying to address these issues, and I think they will help us move the needle. Manufacturers make the devices and we process them, but we both have the same objective – we want that medical device to work perfectly every time that it is used. And for that to happen we need to partner with the manufacturers who must show us how to process that device, walk us through every step, so that every time we process it, we do it correctly and when the surgeon uses it, it is perfect. And that’s good patient care.
HHM: Can certification of sterile processing techs boost compliance with IFUs?
SK: Certification has a bearing on IFU compliance because it provides the “why” in what we do, so when we talk about cleaning a certain way, and validation, etc. techs who go through the certification program understand why all of the steps in the IFUs are necessary. By understanding the “why,” they are inclined to perform the steps correctly. It also helps them to question the process; so if a tech is performing a step in the reprocessing protocol, they can say, “This doesn’t look quite right to me,” and report the issue to their supervisor. In my experience, it’s usually the certified techs who identify the problems and raise the issues; having that additional knowledge that certification provides, they function at a higher level.
HHM: How is technology evolving the sterile processing profession?
SK: Medical devices are so much more complex, and as they continue to evolve, the tools we use in sterile processing must evolve as well to keep up. It’s no longer the flat, hinged, stainless steel instruments we used to process; many more of them are now complex medical devices and it’s not just a simple assemble and package process. There is a great deal of inspection required for these devices –and we must use cleaning validation tools like borescopes. We can use borescopes on almost everything, and not just flexible scopes. A lot of IFUs require lighted magnification and inspection and outline what to look for, so whatever it is we are doing, we must always check the IFUs and make sure we are following them exactly. A key issue is involving the sterile processing department in the healthcare facility’s new product decision-making. A sterile processing leader should look at the product and make sure that the department has the equipment to process the device, as well as adequate time and personnel to do so. We are often not even considered in the product-procurement process, and as a result, we find out too late that the IFU may be difficult or impossible to follow. I have heard many stories where a new medical device is purchased by a hospital and it just sits there because techs can’t process it, they don’t have the tools and equipment specified in the IFU. Also, the cost of processing a new medical device should be taken into consideration in the purchase-related costs of the product. We need the competency of staff as well, to make sure they can process the device. Get us involved at the very beginning.
Newly Revised AORN Guideline on Sterilization Packaging
By Susan Klacik, BS, CRCST, CHL, CIS, ACE, FCS
This column originally appeared in the November 2019 issue of Healthcare Hygiene magazine.
Research has led to many improvements in patient care, including those involving sterilization packaging. Sterilization packaging plays a critical role in patient care. Use of the correct sterilization packaging permits the sterilant from entering and exiting the package, maintains the sterility, and allows for aseptic presentation.
The Association of periOperative Registered Nurses (AORN)’s Guideline for Sterilization Packaging Systems has been relied upon as a best practice since it addresses all activities related to sterilization packaging and has recently undergone key revisions. To update this guideline, AORN assessed peer-reviewed literature published in English from January 2013 until December 2018. The articles were evaluated, rigorously reviewed and appraised for the quality of the evidence. This article will highlight some of the key changes to this guideline.
The sterilization packaging system begins with the pre-purchase evaluation of a packaging system. The guideline provides a listing of considerations such as:
- Product quality assurance testing results
- Compatibility with the intended sterilization method(s) and cycles used within the facility
- Requirements for cleaning according to the instructions for use (IFU) (e.g., laundry for textiles, equipment for cleaning rigid containers)
- Requirements for tracking use
- Method for tracking use
Compatibility with a sterilization process is a primary consideration. This updated guideline includes packaging for sterilization using hydrogen-peroxide combined with ozone, which was recently introduced in the U.S.
Guidance for the preparation for packaging was revised to recommend that packaging for sterilization be performed in an area intended, designed and equipped for sterilization packaging activities, such as the packaging area of the Sterile Processing department (SPD). Prior to packaging, users are advised to verify that instruments and other medical devices have been cleaned, inspected and assembled according to the manufacturer’s IFU.
This updated guideline recommends use of colored or tinted tip protectors for sharp items to protect instrumentation from damage and to protect personnel from injury. The main concern is that colored or tinted tip protectors make it easier for personnel to see, which is important on a sterile field. Clear tip protectors are difficult to see and can pose a risk for a retained surgical item.
The importance of hand hygiene during instrument preparation is also addressed in the guideline. As instrumentation is handled during preparation, there is concern of transferring what is on the hands of the assembler to the instrumentation. Research has shown that contaminants, oils and soils transferred to instruments from the hands of personnel can compromise sterilization. Due to this research, AORN’s updated guideline recommends that personnel who inspect, assemble and package reusable surgical instruments perform hand hygiene within the hour or wear clean gloves to perform these tasks. AORN also recommends performing hand hygiene before handling instruments and medical devices for sterilization.
The updated AORN packaging guideline includes guidance on the packaging of loaned instrumentation, including the recommendation to obtain (from the vendor that provides the instrumentation) sterilization packaging information for loaned instrument sets.
The practice of placing count sheets inside instrument sets has sparked some controversy and there is insufficient evidence for making a recommendation on this practice; therefore, each healthcare organization will need to determine if count sheets may be placed in trays. The decision should consider the limited research available regarding the safety of subjecting toners, inks and various papers to any sterilization method. Chemicals used in the manufacturing of paper, toners and inks pose a theoretical risk of reaction in some sensitized individuals. One research study concluded that the label and toner ink transferred during sterilization was not cytotoxic; however, further study is needed to incorporate a larger sample, various sterilization methods, and instruments of a variety of compositions.
Checking IFU for products used for sterilization is a key principal of sterilization and packaging. AORN’s updated guideline now includes what to look for in the IFU when selecting a single-use, nonwoven sterilization wrapper. In addition, the IFU can provide information for the correct use and maintenance. It is important to note that not all single-use, nonwoven packaging systems are validated for all sterilization methods and cycles.
The guideline also includes recommendations regarding use of corner protectors for wrapped trays. To prevent tears when using flat wrappers for instrument trays, especially those with sharp edges, many facilities use corner guards. The use of corner guards prevents the tears that occur on corners, especially if they are sharp. A tear or hole in a wrapper destroys the integrity of the packaging system since it creates a pathway for microbes to enter a sterilized package and contaminate it. As with all sterilization packaging, it is important to check the IFU of the corner protector manufacturer for information regarding the type of validated sterilization method.
The updated guideline also recommends that users establish and implement a schedule for routine rigid sterilization container inspection, maintenance and repair. Also, new factors have been added regarding what to look for in the container’s IFU that provides information on their correct use.
The complete guideline is available for purchase at www.aorn.org.
Susan Klacik, BS, CRCST, CHL, CIS, ACE, FCS, is clinical director at IAHCSMM.
Recent Survey Reveals Reprocessing Challenges
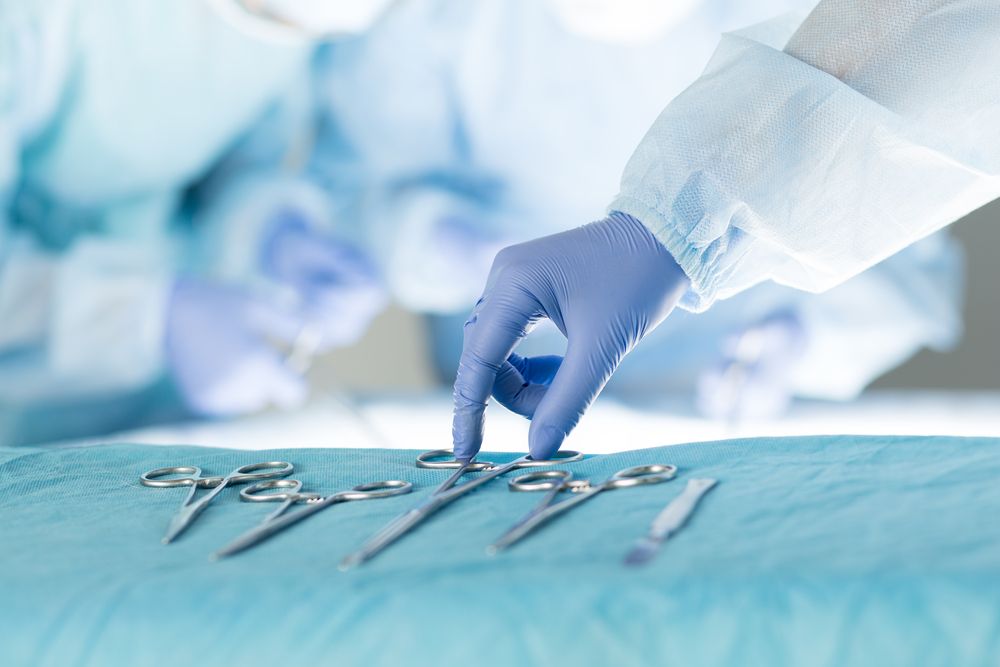
In a survey of the membership of the International Association of Healthcare Central Service Materiel Management (IAHCSMM), just two-thirds believed that manufacturers’ instructions for use (IFU) were understandable. This and other eye-opening findings were unearthed last year by the experts at Ofstead & Associates, who conducted the inquiry to learn which methods are currently used to reprocess endoscopes and what is being done to manage quality. Additionally, the researchers wanted to better understand what challenges IAHCSSM members face related to endoscope reprocessing and gather ideas about potential solutions that could improve the outcomes of reprocessing. A total of 2,334 IAHCSMM members completed the survey.
Regarding reprocessing practices, the researchers found that:
- 69% of respondents follow at least one guideline
- 49% said their facilities follow multiple guidelines.
- 84% had read IFUs for endoscopes; 69% felt the IFUs were understandable or feasible (66%)
- 67% believed that endoscope IFUs were based on scientific evidence.
Regarding the chemistries used for reprocessing flexible endoscopes, the researchers found:
- 27%: OPA
- 22% Hydrogen peroxide
- 21%: Peracetic acid
- 10%: Glutaraldehyde
- 5%: Products with more than one chemistry
Regarding testing the minimum effective concentration (MEC) of HLD before or during each cycle to ensure the HLD is strong enough:
- 79% reported that MEC tests were done every cycle
- 3% tested several times per day
- 14% tested MEC only daily
- 4% never tested MEC or tested it less than once per day
- 51% said that MEC results were documented.
Regarding AER failures:
- 46% observed one or more AER cycle failures in the previous month
- AER cycle failures were frequently attributed to water filter or water flow issues, temperature errors, or leak test failures and channel blockages
Regarding drying and storage of endoscopes:
- 8% acknowledged that they do not dry off the outsides of endoscopes before storage.
For exterior surfaces, survey respondents said they used:
- 59%: single-use lint-free cloths
- 31%: forced air
- 14%: reusable lint-free cloths
- 5%: washcloth
- 4%: paper towels
For endoscope channels:
- 64% indicated they used at least one drying method
- 40% used more than one method (including drip drying)
These drying approaches included:
- 59%: alcohol flush in AER
- 42%: air purge in AER
- 31%: drying cabinet
- 30%: forced-air purge
- 26%: drip dry
- 20%: purge with syringe
Survey respondents reported that their endoscopes were stored the following ways:
- 32%: vertical storage with fan to circulate air
- 29%: vertical storage with ventilation grills, but no fan
- 23%: vertical storage without any ventilation
- 22%: drying cabinet with channel connectors
- 10%: storage bins or drawers
- 7%: other or not sure
Regarding cleaning verification and visual inspection:
- 48% reported using cleaning verification tests to detect residual adenosine triphosphate (ATP), protein or hemoglobin
- 50 % reported using at least one method to visually inspect their endoscopes.
Methods of visual inspection varied, including:
- 36%: visual inspection with the unaided eye
- 18%: visual inspection with a magnifying glass
- 14%: visual inspection with a borescope
- 29% reported performing both visual inspection and cleaning verification
Regarding improving quality, survey respondents suggested:
- Expand opportunities for education and training of technicians and clinicians, including physicians and nurses
- Centralize endoscope reprocessing to one department, to simplify education and competency testing efforts and help ensure each scope is reprocessed by an experienced and competent technician
- Improve working conditions for reprocessing staff personnel who commonly experience health problems and workplace stress that make it difficult to perform their job
- Institute quality management programs that encompass both visual inspection and cleaning verification tests
- Redesign endoscopes and IFUs to simplify the reprocessing process
Survey respondents expressed interest in alternative technologies, including:
- endoscopes that can be disassembled
- sterilizable endoscopes
- single-use/disposable endoscopes
- automation of reprocessing steps
Reference: Ofstead CL, Hopkins KM, et al. Endoscope Reprocessing: Current Practices and Challenges in the Field. Process. 2018.
Sterile Processing Recruiting in an Age of Options: If You Post it, They May Not Come
By Hank Balch
This column originally appeared in the November 2019 issue of Healthcare Hygiene magazine.
There are few better times in the history of sterile processing (SP) to be an experienced professional with a strong resume and industry credentials to back it up. With the U.S. Bureau of Labor Statistics projecting 10 percent to 14 percent growth in the medical device reprocessing field by 2028, all signs point to continued career opportunities for technicians and department leaders.1 This growth, however, brings with it tremendous pressures on hospitals and ambulatory surgery centers to identify, recruit and retain high-quality SP professionals. Many facilities are already feeling the pinch of this competitive job market, seeing their open manager and director positions sit vacant for many months at a time, and spending large percentages of their labor budgets on temporary frontline staffing as the permanent positions go through an extended recruiting process.
There are many contributing factors driving the challenges that we see in today's SP recruiting atmosphere. First, the impact of Baby Boomer retirements is not just a nursing phenomenon but will have similar effects on the shrinking numbers of experienced SP leaders currently in the field. Dr. Peter Buerhaus has projected that a staggering 1 million RNs will retire by 2030 and that “the departure of such a large cohort of experienced RNs means that patient-care settings that depend on RNs will face a significant loss of nursing knowledge and expertise that will be felt for years to come.”2 There is no reason to believe that our departments will be immune to the negative effects of this retirement "brain drain."
Secondly, the known compensation shortcomings of entry-level SP positions are a tremendous barrier for active recruiting out of competitive industries or contiguous geographical areas. With some facilities paying as little as $10 per hour for SP technicians, recruiters end up competing with retail and customer service employers for the same limited pool of candidates. These kinds of limited financial incentives mean that enticing experienced professionals to transfer roles geographically poses an even greater challenge, leaving many facilities with few options but to hire and train on the job.
Thirdly, the market's need for certified and experienced SP professionals 0feeds into the growing ranks of temporary staffing agencies and interim management organizations, creating a kind of self-perpetuating staffing spiral. High-performers who gain their baseline certifications and are willing to travel can make more than double their full-time salaries by becoming SPD "travelers," and department leaders can triple their own salaries by serving as interim managers in facilities who are struggling to recruit a permanent leader. These competitive dynamics will continue to contribute to the vacancy rates of many departments until permanent compensation models are able to catch up to the temporary/interim staffing rates.
Lastly, many hospital recruiters do not understand what they should be looking and recruiting for in a potential SP technician. While the recruiting structures vary, it is not uncommon for so-called "clinical recruiters" to cover recruiting for perioperative staff such as RNs, surgical technicians and anesthesia personnel, but recruiting responsibility for sterile processing is placed under "non-clinical recruiting" which includes other departments such as dietary, environmental services and linen services. Inherent in this structure is the idea that what makes a great candidate for a dietary role might also make a great candidate for SP positions. For many reasons, categorizing Sterile Processing departments as non-clinical recruiting pools leads to an unhelpful narrowing of potential candidates and loss of strategic focus for where these professionals can and should be sourced from.
As dire as this situation may sound and as frustrating as it may feel to experience it as a department leader, there are certain insights that can be leveraged to find success in sourcing high-quality SP candidates.
Department leaders must realize they must take an active role in the recruiting process if they want to have the greatest success with opening up a pipeline of potential hires. Relying on a hospital recruiter who does not have the subject-matter expertise of our field to identify these professionals is really asking them to hit a moving target. Even if they want to be successful, they will need your help to clarify exactly what does and does not make a great frontline technician or shift leader.
Related to this, department leaders need to get creative about where and how their candidates are sourced. If “recruiting” in your hospital simply means posting the job on the hospital website, then you should not be surprised that your applicant pool is primarily internal candidates looking to transfer from another department, instead of reaching the broader audience outside of the facility. Taking the time to post fliers at a local community college, healthcare trade school and coffee shops can extend your reach dramatically. Partnering with local organizations such as refugee placement and military veteran groups can also broaden your potential network for candidates with previous related experience in various contexts.
While you may have limited ability to directly impact your compensation rates, you may have success in wrapping in tuition reimbursement packages to cover the costs of SP training programs for staff and/or traditional college degrees, both of which can provide a competitive edge in recruiting.
Confronting staffing challenges will not be easy, and few solutions look to be the silver bullet to solve all the recruiting issues facing our teams. However, knowing the factors that have led to our current state gives us the insight necessary to develop strategies that can have real success. The days of posting a job and waiting for a flood of quality applicants is over. What the future looks like is up to you.
Hank Balch is an internationally recognized thought leader in the sterile processing industry, as well as podcast host, and founder of Beyond Clean.
References:
- Bureau of Labor Statistics. 2018 wage dataand 2018-2028 employment projections. https://www.onetonline.org/link/details/31-9093.00
- Buerhaus PI, et al. Four Challenges Facing the Nursing Workforce in the U.S. http://healthworkforcestudies.com/images/JNR0717_40-46_Buerhaus.pdf
The Missing Science of Sterile Processing: On Credentials and Real Improvement
By Hank Balch
This column originally appeared in the October 2019 issue of Healthcare Hygiene magazine.
Microbiology, chemistry, physics.
Some of us took introduction classes to these subjects in high school, a few went on to take a semester or two in college, but for many sterile processing professionals across the country these hard sciences are mostly relegated to a chapter or two in a certification textbook. After all, do we really need to know chemistry to do our jobs well? How important can physics really be to an SPD technician? Being able to speak the language of microbiology couldn't be that important to surgical instrument reprocessing, could it?
Does anyone know what a biocatalyst is? Or what it has to do with the decontamination process? At the end of the day, do these questions even matter?
In fact, every single one of us in the industry know the answer to this question is "Yes, science matters!" But the near universal absence of credentialed scientists employed in sterile processing departments tells a very different story. Unless your department is led by a B.S. in biology or employed solely by team members with B.S. degrees in chemistry, your exposure to the true science behind how your sterile processing department actually interact with chemistry, physics, and microbiology is limited to whatever self-study you pursued under your own initiative. Admittedly, many CS/SPD leaders and technicians have taken these extra steps to fill in the science knowledge gaps, but the fact that they had to go over and above the industry standards should tell us something very important about the current "industry standards" – namely, they are way too low.
Breaking the Chains and Changing the World
So, you can name the biological spore used in steam sterilization testing -- geobacillus stearothermophilus … so what? Why that spore instead of another? Why do we use spore testing at all? Is there a better way to measure sterility assurance and how would we know? If you ask sterile processing professionals across the country, we want to know the answers to these questions. We know it's important. And we know that our ability to holistically grasp these categories, and do so in a creative, innovative way, will move the industry of CS-improvement firmly in-house, rather than being dependent upon external vendors (who, by the way, employ chemists, physicists and microbiologists to create their nifty new products).
As generations of us learned in Schoolhouse Rock!, “knowledge is power” -- and in the case of the hard sciences, knowledge is also the engine of innovation, the catalyst for product changes, and the great differentiator in industry compensation. CS/SPD professionals talk a lot about facility pay practices and how our counterparts in the OR make somewhere close to 200 percent to 300 percent more than our teams, but what is often left out of the equation is the two- to three-times more education RNs and BSNs have as compared to CS/SPD technicians. That need not be so. There is another way.
The Great Educational Leap: Know Ye the Truth
So, what can deliver our industry from the basement of healthcare compensation, sluggish career growth, and token respect? I believe one of the best ways to get our teams to where we want to be and where our patients need us to be is to take a great educational leap in sterile processing. The recent success we have seen over the last decade in the surge of industry certification through the IAHCSMM and CBSPD organizations (not only the standard certification, but also secondary and tertiary certifications as well) has sparked a deep hunger among CS professionals for continued growth. What we have not yet seen is a dramatic impact of these certifications upon total compensation and demonstrable quality metrics in the country’s CS departments. In other words, even with more credentials, there’s still something missing.
I believe that missing link has to do, in part, with the need to create a science-heavy, higher-education career track that not only prepares CS professionals for every aspect of the role, but also provides our people with recognized degrees that translate into competitive pay-grades as compared with our OR peers. Simply put, if we really want to solve the pay/quality issues of our industry, it will take more mere certifications – even if they are mandated in all 50 states. One certification course doth not an expert make.
And to be fair, these certification bodies do not claim the title of “expert” for their certification holders. Instead the language is usually something like “providing a baseline knowledge” of industry standards. And for what it’s worth, that is a noble and needed cause. But is it enough? Is baseline knowledge enough for the systemic quality issues in our industry? Is one textbook course going to shake the compensation tree in the ways that we desperately need to drive these departments forward?
I think we all know the answers to this. But what are we going to do about it?
Hank Balch is founder and president of Beyond Clean.