The National Institutes of Health has awarded $3.8 million to Texas Biomedical Research Institute to further develop a promising HIV vaccine candidate that stops the virus upon entry, before it begins rapidly spreading throughout the body.
Texas Biomed professor Marie-Claire Gauduin, PhD, has been developing this novel vaccine approach for more than 10 years with the support of NIH grants, including one for out-of-the-box, yet feasible ideas. The new four-year grant will enable Gauduin and her team to build on promising results in nonhuman primates and delve deeper into how the vaccine works on molecular and genetic levels. She will also gather more robust data about safety, efficacy and different delivery methods.
“I am excited to move forward, and hope to get to the preclinical stage for human trials,” Gauduin says.
An effective HIV vaccine has eluded the biomedical community for decades. Part of the challenge is that the virus spreads throughout the body within days of initial infection, reaching peak viral levels within two weeks.
Gauduin thought, then, why not try to stop HIV where it enters, before it infects individual cells and gains a foothold in the rest of the body?
“I had this idea as a postdoc,” Gauduin says. “I thought it had to be naïve because nobody was talking about it. It was so obvious and simple to me; I thought someone would have already done it.”
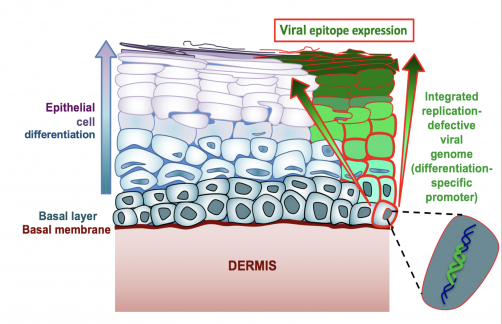
Gauduin’s vaccine specifically targets the interior lining of the vagina and rectum, called the mucosal epithelium, which is where the virus is most likely to enter the body. The vaccine stimulates the production of antibodies specifically in these areas. Much like a bouncer checking for fake IDs just inside the door, or sentries along a castle wall, the antibodies are well positioned to quickly respond and block the virus from proceeding any further.
The vaccine does not target just any cell in the epithelium, but is designed to enter the basal cells that make up the base layer of the lining. These basal cells are “mother” cells, generating replacement cells as needed in the vagina and rectum. For females, the epithelial lining builds up and is sloughed off in each menses cycle; while the rectum lining remains more constant and new cells are generated as old ones die.
“The idea is that as long as the vaccine is in the mother cells, it will be passed on and be present in all new epithelial cells in these regions,” Gauduin says. “I did not think it would work so well, but it did!”
The vaccine, which has been patented, is a live attenuated vaccine, meaning it is based on the full genetic code of HIV, but has certain parts cut or removed so it is not harmful. Live attenuated vaccines are used for several diseases, such as smallpox and yellow fever, but have failed with HIV in the past because the virus mutated enough over decades to regain its potency.
To prevent this from happening in her vaccine, Gauduin is putting multiple safeguards in place. She cuts the genetic code in key places related to viral function. Notably, she removed the vaccine virus’s ability to replicate and spread. The so-called “single-cycle” vaccine virus can enter cells, but then becomes trapped, unable to leave. The cells signal that they contain the vaccine virus to the immune system, which then makes antibodies. The antibodies do not attack the cells with the vaccine, likely because the immune system recognizes those cells are native.
In her previous studies, Gauduin found that vaccinated nonhuman primates took much longer to become infected with SIV, the monkey equivalent of HIV, than the unvaccinated group. While they eventually became infected after repeat exposures, the vaccinated animals completely controlled the infection – there were no signs or symptoms of disease and no detectable levels of SIV in the blood, which is the gold standard for measuring infection. One animal continued to control infection for more than three years with no side effects.
“So potentially we have developed a therapeutic vaccine that can control HIV with one dose, no boosting required, compared to daily antiretroviral pills,” Gauduin says.
The next round of studies will involve a larger group of animals, which is required to prove safety and efficacy before the vaccine can move forward to human clinical trials. Gauduin will gather more data about where the vaccine goes in the body and how it controls infection.
Currently, the vaccine is delivered directly to the mucosal lining by liquid drops. Gauduin will explore other delivery methods that may prove more efficient, which will be critical for making it practical for mass immunization in developing countries.
Source: Texas Biomedical Research Institute