A promising strategy to reduce the impact of viral infectious diseases, such as influenza, is the use of attenuated, live viruses as vaccines. However, the usefulness of traditional live-attenuated virus vaccines has often been limited by suboptimal immunogenicity, safety concerns, or cumbersome manufacturing processes and techniques. In addition, immune escape due to rapid viral evolution poses a further challenge for traditional influenza vaccines.
Recently, a research team led by professor Si Longlong from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has proposed a new live-attenuated influenza vaccine approach — generating proteolysis-targeting chimeric (PROTAC) influenza A virus as a live-attenuated vaccine by utilizing the endogenous ubiquitin-proteasome system of host cells to degrade viral proteins.
The results were published in Nature Biotechnology on July 4.
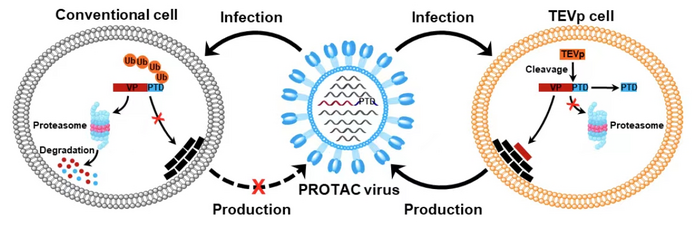
Given that virus replication depends on virally encoded proteins, manipulation of viral protein stability by utilizing the protein degradation machinery of the host cell may represent a potential approach to switch the viral life cycle on and off for vaccine development. Thus, the researchers designed proteolysis-targeting chimeric (PROTAC) viruses by fusing a conditionally removable proteasome-targeting domain (PTD) to influenza viral proteins.
The PTD was designed to contain a proteasome-targeting peptide and a tobacco etch virus cleavage site (TEVcs) linker. It was used to selectively induce proteasomal degradation of viral proteins of interest; however, the TEVcs linker could be selectively cleaved by the tobacco etch virus protease (TEVp) to separate the viral proteins from the PTD, thus sparing them from degradation.
Accordingly, the researchers engineered the genome of influenza A viruses in TEVp-expressing stable cell lines engineered for virus production to introduce the conditionally removable PTD, generating fully infective PROTAC viruses that were live-attenuated by the host protein degradation machinery upon infection.
In mouse and ferret models, PROTAC viruses were sufficiently attenuated but able to elicit robust and broad humoral, mucosal, and cellular immunity. As a result, they provided broad protection against homologous and heterologous virus challenges.
"This PROTAC vaccine technology could also be useful for generating live-attenuated vaccines against other types of pathogens," said Longlong.