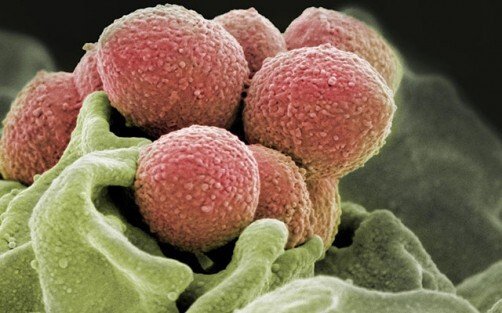
Many disease-causing bacteria like Streptococcus pneumoniae (S. pneumoniae) are encased in a sugar layer called the capsular polysaccharide (CPS). This layer is often essential for infections. In a ground-breaking discovery by scientists from the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), features of the CPS that help the bacteria to colonize the human respiratory tract were identified. The research showed that the structures of the CPS capsule and its types of linkages and combinations matter greatly in allowing the bacteria to better attach and survive on the lining of the upper and lower human respiratory tracts.
To challenge the widely-held notion that structurally diverse CPS capsules in S. pneumoniae perform the same function in promoting bacterial colonization, the team, led by assistant professor Chris Lok-To Sham and graduate student, Jade Chun Ye-Yu, from the Infectious Diseases Translational Research Program at NUS Medicine, constructed bacterial mutants displaying one of the 84 types of CPS found in S. pneumoniae. The mutants were then introduced to respiratory cells to investigate their abilities to bind to the respiratory tracts. Using a molecular barcode to distinguish the strains, the team examined whether varying CPS in these mutants would affect binding on the nasal and bronchial cells.
The results showed that the CPS with rhamnose sugar residues bound poorly to the airway cells, while CPS with glycan motifs bound strongly. The experiment demonstrated that the structural configurations and the types of CPS play important roles in the strength of attachment and survival on the human airway.
“In the past, scientists recognized that the proteins found in bacteria are not by chance, and they do serve a purpose. Bacteria have shown a preference for certain types of sugars on their capsules, and a specific linkage of sugars. Our research proves that some of these combinations benefit the bacteria because they aid in colonizing the human respiratory tract. This finding will shed more light on the range of CPS types to be included in future vaccines, as current vaccines against S. pneumoniae do not cover the many types of CPS produced by the bacteria,” added Jade Chun, who is also from the Department of Microbiology and Immunology at NUS Medicine.
S. pneumoniae is a major driver of pneumonia, septicemia, and meningitis. Collectively, these infections are among the leading causes of morbidity and mortality in the elderly and young children. To fight against these deadly infections, pneumococcal vaccines are administered to stimulate antibody production to the CPS. However, the bacteria can manipulate their CPS structure to evade these antibodies. This biochemical warfare results in more than a hundred types of CPSs produced by S. pneumoniae, which increases the challenge of producing effective vaccines. While the diversity of CPS is well appreciated, what actually makes the CPS a lethal weapon for the bacteria remains unclear.
The findings have been published in The Proceedings of the National Academy of Sciences (PNAS), a peer-reviewed journal of the National Academy of Sciences (NAS).