Many individuals infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to experience debilitating clinical symptoms, years after the initial infection. Such clinical symptoms that persist for longer than 12 weeks and cannot be attributed to an alternate diagnosis are referred to as post COVID-19 syndrome or post-acute sequelae of SARS-CoV-2 infection (PASC), or long-COVID.
Explaining the complexity of this lingering condition, Dr. Jiuyang Xu, a physician researcher at China–Japan Friendship Hospital in Beijing, China, says, “Long-COVID is highly heterogeneous in its clinical presentation. More than 200 different symptoms are ascribed to long-COVID. The most commonly reported complaints include shortness of breath, fatigue, brain fog, anosmia, hair loss, sexual dysfunction, and sleep alteration.”
A follow-up study among patients in Wuhan, China, infected with the original strain of SARS-CoV-2 virus, reports symptoms persisting even two years post infection. Now, Xu and his colleagues reviewed published evidence to describe the prevailing symptoms and possible mechanisms of pathogenesis underlying long COVID. This paper was published in the Chinese Medical Journal Pulmonary and Critical Care Medicine on Dec. 6, 2023.
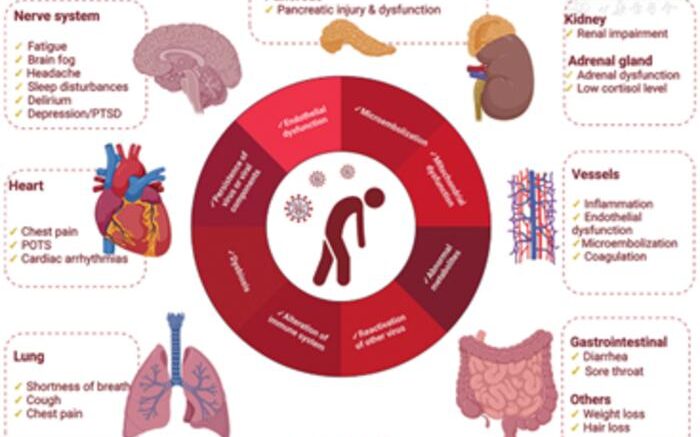
It was noted that both severe and mild infections could result in long-COVID. Older persons, unvaccinated, and those infected with the original SARS-CoV-2 variant show increased incidence of post-acute sequelae. Although the pathogenesis of long-COVID remains unclear, the mechanisms proposed include immune system alterations, chronic inflammation triggered by persisting residual viral components, endothelial dysfunction, microembolization, mitochondrial dysfunction, abnormal metabolites, reactivation of pre-existing chronic viral infection, dysbiosis of microbiota, and unrepaired tissue damage.
In patients with long COVID, several changes were noted in the SARS-CoV-2 virus-specific adaptive- and innate immune responses. The cluster of differentiation (CD)4+ and CD8+ T cells exhibited an exhausted (programmed cell death protein 1 (PD-1)-expressing)/senescent (CD57-expressing) state, and CD4+ regulatory T cells were perturbed, indicative of chronic immune activation. High antiviral cytotoxicity in CD8+ T cells, augmented granzyme B and interferon gamma (IFN-𝛾) production, indicated unresolved inflammation. Significant increase in numbers of monocytes, mast cells, and NK cells was also noted. The cytokine profile of long COVID included high serum levels of pro-inflammatory interleukin (IL)-17 and IL-6, and low levels of IL-4 and anti-inflammatory IL-10. These were proposed as potential therapeutic targets for long COVID.
Moreover, the review reveals that long-COVID is a multi-system disorder. Extreme obesity and lipid metabolism disorders increased the risk of long-COVID. Loss of mitochondrial membrane potential indicative of mitochondrial dysfunction, adrenal gland dysfunction due to reduced cortisol, and loss of kidney function, were all noted in patients with long-COVID. In these patients, activation of the absent in melanoma 2 (AIM2) receptor in circulating cells and the release of IL-1α, IFN-α, and transforming growth factor β lead to lung fibrosis. Prolonged persistence of coronavirus in the cardiomyocytes, endothelium, and macrophages can lead to myo-endocarditis. Moreover, arterial wall stiffening and endothelial dysfunction can contribute to cardiac dysfunction in long-COVID. Elevated D-dimer levels explain the large amyloid clots observed in the plasma of patients with long-COVID.
Persistent circulating SARS-CoV-2 spike protein detected in the plasma of patients also increased the risk of long-COVID. Intriguingly, reactivation of Epstein–Barr virus was observed in patients with long COVID fatigue.
Gut microbiome analysis identified a multi-kingdom (bacteria, virus, and fungi) cluster, with prognostic value, that significantly correlated with risk of long-COVID. Patients with higher antinuclear antibody titers were found to have significantly higher proportions of neurocognitive symptoms. Additionally, chronic symptoms of fatigue are attributed to brainstem dysfunction induced by the tropism of SARS-CoV-2. Damage to the autonomic nervous system reported in long-COVID correlates with psychiatric disorders and suicide risk.
The prevalence of long-COVID continues to be observed among us in the form of new emerging variants of SARS-CoV-2 (such as the omicron variant), and the response will need close monitoring. Dr. Yan Liu, the first author of the review, concludes, “Although we are gradually accumulating evidence regarding long COVID, whether omicron causes persisting symptoms and whether the mechanisms are similar to those of previous variants are still unknown. Much work remains to more clearly understand the mechanisms of long COVID.”
Source: China–Japan Friendship Hospital