Researchers across the globe continue their endeavors to improve our understanding of the mechanisms underlying COVID-19 infection and the pneumonia and lung damage occasionally associated with it. As part of these endeavors, researchers from Charité, the Berlin Institute of Health at Charité (BIH), the Max Delbrück Center for Molecular Medicine (MDC), the Robert Koch Institute, and Freie Universität Berlin have analyzed the propagation of, and immune activation by, SARS-CoV-2 viruses inside human lungs. Specifically, the researchers focused on cells within the alveoli and alveolar macrophages. The latter are phagocytic cells of the innate immune system which clean the lungs by ingesting and eliminating foreign particles, including infective pathogens such as viruses and bacteria.
Led by Dr. Andreas Hocke of Charité’s Department of Infectious Diseases and Respiratory Medicine, the team of researchers found that SARS-CoV-2 infects very few of the epithelial cells lining the alveolar surface, meaning that it causes very little direct tissue damage. In this respect, the virus differs significantly from both MERS-CoV and influenza viruses. Using comprehensive spectral microscopy-based analyses, the researchers were also able to show that only very few alveolar epithelial cells possess ACE2 receptors, which are required by SARS-CoV-2 to gain entry into host cells.
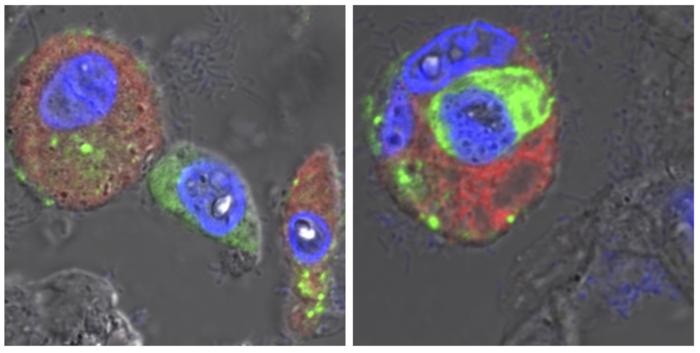
“Using human lungs and lung organoids – models of human alveoli developed using stem cells harvested from human lung tissue – we were able to show that SARS-CoV-2 is directly dependent on its receptor. By doing so, we were able to exclude other, alternative receptors,” explains the study’s first author, Dr. Katja Hönzke of Charité’s Department of Infectious Diseases and Respiratory Medicine. When large quantities of SARS-CoV-2 reach the alveoli from the upper respiratory tract, these do not propagate widely within the lungs’ epithelial cells, as is often the case in other severe viral infections. Instead, they are subject to ingestion by macrophages.
“Using detailed bioinformatics analyses and COVID-19 autopsy samples, we observed that the macrophages change after ingesting coronaviruses,” says co-first author Dr. Benedikt Obermayer-Wasserscheid of the BIH. These changes in turn trigger a range of reactions which are associated with pneumonia. The macrophages release inflammatory messengers and can occasionally set off severe inflammatory cascades. The researchers also observed that the virus does not replicate inside these immune cells.
Explaining the researchers’ findings, Hocke says, “Our study suggests that severe lung injury in COVID-19 is more likely to be due to macrophage-induced immune activation than direct, virus-induced damage to the alveoli. As such, it makes a major contribution to our understanding of disease progress during the early phase of potential COVID-19 pneumonia and shows why, in contrast to MERS coronaviruses, most cases of SARS-CoV-2 infection show relatively moderate disease severity.” It is therefore safe to assume that, in most cases of SARS-CoV-2 infection, local immune mechanisms within human lung tissues are effective at removing the virus and limiting inflammatory response. Where this fails to happen – something which may be affected by individual risk factors – the infection may, in rare cases, prove severe and even fatal. Prof. Hocke continues: “The lung models we used provide an excellent example of how human cell-based alternatives to animal models are particularly useful in zoonotic diseases research. We were able to do this thanks to our close collaboration with Charité 3R, a facility dedicated to developing alternatives to animal-based research.”
Subsequent research will focus on patient-derived organoid models, which will be used for a more detailed study of how general risk factors such as age, gender, concomitant disease and different medicines affect the activation of the immune response. This knowledge may then enable the identification of possible treatment approaches targeting the immune system.
Reference: Hönzke K, Obermayer B, Mache C, et al. Human lungs show limited permissiveness for SARS-CoV-2 due to scarce ACE2 levels but virus-induced expansion of inflammatory macrophages. European Respiratory Journal (2022). doi: 10.1183/13993003.02725-2021